Biomaterials ( IF 12.8 ) Pub Date : 2018-02-23 , DOI: 10.1016/j.biomaterials.2018.02.033 Ting Kang , Yukun Huang , Qianqian Zhu , Hao Cheng , Yuanyuan Pei , Jingxian Feng , Minjun Xu , Gan Jiang , Qingxiang Song , Tianze Jiang , Hongzhuan Chen , Xiaoling Gao , Jun Chen
|
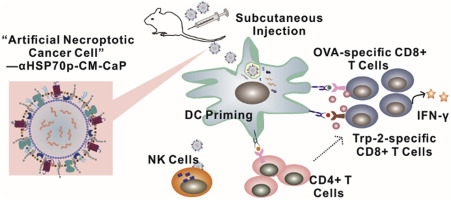
Recent breakthroughs in cancer immunotherapy offer new paradigm-shifting therapeutic options for combating cancer. Personalized therapeutic anti-cancer vaccines training T cells to directly fight against tumor cells endogenously offer tremendous benefits in working synergistically with immune checkpoint inhibitors. Biomimetic nanotechnology offers a versatile platform to boost anticancer immunity by efficiently co-delivering optimized immunogenic antigen materials and adjuvants to antigen presenting cells (APC). Necroptotic tumor cells can release danger associated molecule patterns (DAMPs) like heat shock proteins, being more immunogenic than naïve tumor cells. Here, nano-size “artificial necroptotic cancer cell” (αHSP70p-CM-CaP) composing of phospholipid bilayer and a phosphate calcium core was designed as a flexible vaccine platform for co-delivering cancer membrane proteins (CM), DAMPs signal-augmenting element α-helix HSP70 functional peptide (αHSP70p) and CpG to both natural killer (NK) cells and APC. Mechanically, immunogenic B16OVA tumor cells membrane-associated antigens and αHSP70p were reconstituted in artificial outer phospholipid bilayer membrane via one-step hydration and CpG encapsulated in the phosphate calcium core. The resulted αHSP70p-CM-CaP exhibited 30 nm in diameter with the immunogenic membrane proteins reserved in the particles to produce synergistic effect on bone marrow derived dendritic cells maturation and antigen-presentation. Following αHSP70p-CM-CaP vaccination, efficient lymph node trafficking and multi-epitope-T cells response was observed in mice. Vitally, αHSP70p-CM-CaP was also able to induce expansion of IFN-γ-expressing CD8+ T cells and NKG2D+ NK cells subsets. Most promisingly, αHSP70p-CM-CaP vaccination led to the killing of target cells and tumor regression in vivo when combined with anti-PD-1 antibody treatment on mice B16OVA melanoma models. Altogether, we demonstrated proof-of-concept evidence for the feasibility, capability and safety of a nanovaccine platform towards efficient personalized anticancer application.
中文翻译:

死灵性癌细胞模仿纳米疫苗通过量身定制的免疫刺激方式增强抗肿瘤免疫力
癌症免疫疗法的最新突破为抗击癌症提供了新的范式转变疗法。训练T细胞内源性直接对抗肿瘤细胞的个性化治疗性抗癌疫苗在与免疫检查点抑制剂协同工作方面提供了巨大的好处。仿生纳米技术通过将优化的免疫原性抗原材料和佐剂有效地共同递送至抗原呈递细胞(APC),提供了增强抗癌免疫力的多功能平台。坏死性肿瘤细胞可以释放与危险相关的分子模式(DAMP),例如热休克蛋白,其免疫原性要比单纯的肿瘤细胞高。这里,由磷脂双层和磷酸钙核心组成的纳米级“人工坏死性癌细胞”(αHSP70p-CM-CaP)被设计为灵活的疫苗平台,用于共同递送癌膜蛋白(CM),DAMPs信号增强元件α-螺旋HSP70功能肽(αHSP70p)和CpG传递给自然杀伤(NK)细胞和APC。在机械上,通过一步水合将免疫原性B16OVA肿瘤细胞膜相关抗原和αHSP70p重构在人工外部磷脂双层膜中,并将CpG封装在磷酸钙核心中。所得的αHSP70p-CM-CaP直径为30 nm,其免疫原性膜蛋白保留在颗粒中,从而对源自骨髓的树突状细胞成熟和抗原呈递产生协同作用。接种αHSP70p-CM-CaP疫苗后,在小鼠中观察到有效的淋巴结运输和多表位-T细胞反应。实际上,αHSP70p-CM-CaP还能够诱导表达IFN-γ的CD8扩增+ T细胞和NKG2D + NK细胞子集。最有希望的是,当在小鼠B16OVA黑色素瘤模型上联合抗PD-1抗体治疗时,αHSP70p-CM-CaP疫苗接种可导致体内靶细胞杀伤和肿瘤消退。总而言之,我们证明了纳米疫苗平台朝着有效的个性化抗癌应用的可行性,能力和安全性的概念验证证据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号