PLoS Pathogens ( IF 5.5 ) Pub Date : 2018-02-22 , DOI: 10.1371/journal.ppat.1006837 Jarrod J Mousa 1 , Elad Binshtein 2 , Stacey Human 3, 4 , Rachel H Fong 5 , Gabriela Alvarado 6 , Benjamin J Doranz 5 , Martin L Moore 3, 4 , Melanie D Ohi 2 , James E Crowe 1, 6, 7
|
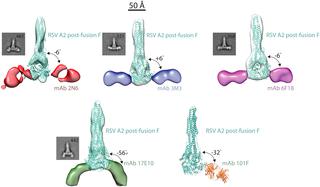
Respiratory syncytial virus (RSV) is a major human pathogen that infects the majority of children by two years of age. The RSV fusion (F) protein is a primary target of human antibodies, and it has several antigenic regions capable of inducing neutralizing antibodies. Antigenic site IV is preserved in both the pre-fusion and post-fusion conformations of RSV F. Antibodies to antigenic site IV have been described that bind and neutralize both RSV and human metapneumovirus (hMPV). To explore the diversity of binding modes at antigenic site IV, we generated a panel of four new human monoclonal antibodies (mAbs) and competition-binding suggested the mAbs bind at antigenic site IV. Mutagenesis experiments revealed that binding and neutralization of two mAbs (3M3 and 6F18) depended on arginine (R) residue R429. We discovered two R429-independent mAbs (17E10 and 2N6) at this site that neutralized an RSV R429A mutant strain, and one of these mAbs (17E10) neutralized both RSV and hMPV. To determine the mechanism of cross-reactivity, we performed competition-binding, recombinant protein mutagenesis, peptide binding, and electron microscopy experiments. It was determined that the human cross-reactive mAb 17E10 binds to RSV F with a binding pose similar to 101F, which may be indicative of cross-reactivity with hMPV F. The data presented provide new concepts in RSV immune recognition and vaccine design, as we describe the novel idea that binding pose may influence mAb cross-reactivity between RSV and hMPV. Characterization of the site IV epitope bound by human antibodies may inform the design of a pan-Pneumovirus vaccine.
中文翻译:

人抗体识别肺病毒融合蛋白上的抗原位点 IV
呼吸道合胞病毒 (RSV) 是一种主要的人类病原体,可感染大多数两岁以下的儿童。RSV 融合 (F) 蛋白是人类抗体的主要靶标,它具有几个能够诱导中和抗体的抗原区域。RSV F的融合前和融合后构象均保留抗原位点IV。抗原位点IV的抗体已被描述为结合并中和RSV和人偏肺病毒(hMPV)。为了探索抗原位点 IV 结合模式的多样性,我们生成了一组四种新的人单克隆抗体 (mAb),竞争结合表明 mAb 与抗原位点 IV 结合。诱变实验表明,两种 mAb(3M3 和 6F18)的结合和中和取决于精氨酸 (R) 残基 R429。我们在该位点发现了两种不依赖 R429 的 mAb(17E10 和 2N6),它们中和了 RSV R429A 突变株,其中一种 mAb(17E10)中和了 RSV 和 hMPV。为了确定交叉反应的机制,我们进行了竞争结合、重组蛋白诱变、肽结合和电子显微镜实验。已确定人交叉反应性 mAb 17E10 以类似于 101F 的结合位姿与 RSV F 结合,这可能表明与 hMPV F 的交叉反应性。提供的数据为 RSV 免疫识别和疫苗设计提供了新概念,如我们描述了结合姿势可能影响 RSV 和 hMPV 之间的 mAb 交叉反应性的新观点。人抗体结合的位点 IV 表位的表征可能为泛肺病毒疫苗的设计提供信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号