Analytical and Bioanalytical Chemistry ( IF 3.8 ) Pub Date : 2018-02-22 , DOI: 10.1007/s00216-018-0924-y Jimmy Ly 1, 2, 3 , Noel S Ha 1, 2 , Shilin Cheung 2, 4 , R Michael van Dam 1, 2
Miniaturized synthesis of positron emission tomography (PET) tracers is poised to offer numerous advantages including reduced tracer production costs and increased availability of diverse tracers. While many steps of the tracer production process have been miniaturized, there has been relatively little development of microscale systems for the quality control (QC) testing process that is required by regulatory agencies to ensure purity, identity, and biological safety of the radiotracer before use in human subjects. Every batch must be tested, and in contrast with ordinary pharmaceuticals, the whole set of tests of radiopharmaceuticals must be completed within a short-period of time to minimize losses due to radioactive decay. By replacing conventional techniques with microscale analytical ones, it may be possible to significantly reduce instrument cost, conserve lab space, shorten analysis times, and streamline this aspect of PET tracer production. We focus in this work on miniaturizing the subset of QC tests for chemical identity and purity. These tests generally require high-resolution chromatographic separation prior to detection to enable the approach to be applied to many different tracers (and their impurities), and have not yet, to the best of our knowledge, been tackled in microfluidic systems. Toward this end, we previously explored the feasibility of using the technique of capillary electrophoresis (CE) as a replacement for the “gold standard” approach of using high-performance liquid chromatography (HPLC) since CE offers similar separating power, flexibility, and sensitivity, but can readily be implemented in a microchip format. Using a conventional CE system, we previously demonstrated the successful separation of non-radioactive version of a clinical PET tracer, 3′-deoxy-3′-fluorothymidine (FLT), from its known by-products, and the separation of the PET tracer 1-(2′-deoxy-2′-fluoro-β-D-arabinofuranosyl)-cytosine (D-FAC) from its α-isomer, with sensitivity nearly as good as HPLC. Building on this feasibility study, in this paper, we describe the first effort to miniaturize the chemical identity and purity tests by using microchip electrophoresis (MCE). The fully automated proof-of-concept system comprises a chip for sample injection, a separation capillary, and an optical detection chip. Using the same model compound (FLT and its known by-products), we demonstrate that samples can be injected, separated, and detected, and show the potential to match the performance of HPLC. Addition of a radiation detector in the future would enable analysis of radiochemical identity and purity in the same device. We envision that eventually this MCE method could be combined with other miniaturized QC tests into a compact integrated system for automated routine QC testing of radiopharmaceuticals in the future.
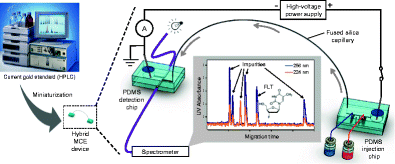
Miniaturized quality control (QC) testing of batches of radiopharmaceuticals via microfluidic analysis. The proof-of-concept hybrid microchip electrophoresis (MCE) device demonstrated the feasibility of achieving comparable performance to conventional analytical instruments (HPLC or CE) for chemical purity testing.
中文翻译:

通过微芯片电泳对放射性药物的化学特性和纯度进行小型化分析
正电子发射断层扫描 (PET) 示踪剂的小型化合成有望提供众多优势,包括降低示踪剂生产成本和提高多种示踪剂的可用性。虽然示踪剂生产过程的许多步骤已经小型化,但用于质量控制 (QC) 测试过程的微型系统的开发相对较少,监管机构要求该过程确保放射性示踪剂在使用前的纯度、特性和生物安全性在人类受试者中。每一批都必须进行检测,与普通药品相比,放射性药品的整套检测必须在短时间内完成,以尽量减少放射性衰变造成的损失。通过用微量分析技术取代传统技术,可以显着降低仪器成本、节省实验室空间、缩短分析时间并简化 PET 示踪剂生产的这一方面。我们的工作重点是小型化化学特性和纯度的 QC 测试子集。这些测试通常需要在检测之前进行高分辨率色谱分离,以使该方法能够应用于许多不同的示踪剂(及其杂质),并且据我们所知,尚未在微流体系统中得到解决。为此,我们之前探讨了使用毛细管电泳 (CE) 技术替代高效液相色谱 (HPLC) 的“金标准”方法的可行性,因为 CE 提供类似的分离能力、灵活性和灵敏度,但可以很容易地以微芯片格式实现。我们之前使用传统的 CE 系统演示了临床 PET 示踪剂的非放射性版本 3'-脱氧-3'-氟胸苷 (FLT) 与其已知副产物的成功分离,以及 PET 示踪剂的分离1-(2′-脱氧-2′-氟-β-D-阿拉伯呋喃糖基)-胞嘧啶 (D-FAC) 的 α-异构体,其灵敏度几乎与 HPLC 一样好。在此可行性研究的基础上,我们在本文中描述了通过使用微芯片电泳 (MCE) 来小型化化学特性和纯度测试的首次努力。全自动概念验证系统包括样品注射芯片、分离毛细管和光学检测芯片。使用相同的模型化合物(FLT 及其已知副产品),我们证明了样品可以注射、分离和检测,并显示出与 HPLC 性能相匹配的潜力。未来添加辐射探测器将能够在同一设备中分析放射化学特性和纯度。我们预计,最终这种 MCE 方法可以与其他小型化 QC 测试结合成一个紧凑的集成系统,用于将来对放射性药物进行自动化常规 QC 测试。

通过微流体分析对放射性药物批次进行小型化质量控制 (QC) 测试。概念验证混合微芯片电泳 (MCE) 设备证明了在化学纯度测试方面实现与传统分析仪器(HPLC 或 CE)相当的性能的可行性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号