当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Relaxation in Solutions of Monoclonal Antibodies
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-22 00:00:00 , DOI: 10.1021/acs.jpcb.7b11053 Gang Wang 1 , Zsigmond Varga 1 , Jennifer Hofmann 1 , Isidro E. Zarraga 2 , James W. Swan 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-22 00:00:00 , DOI: 10.1021/acs.jpcb.7b11053 Gang Wang 1 , Zsigmond Varga 1 , Jennifer Hofmann 1 , Isidro E. Zarraga 2 , James W. Swan 1
Affiliation
|
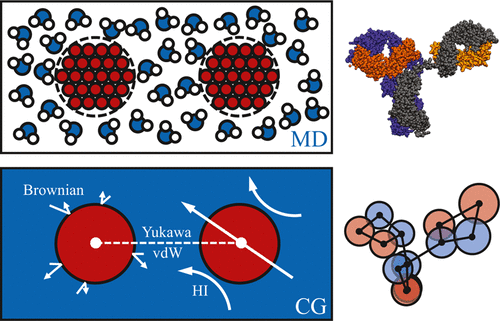
Reversible self-association of therapeutic antibodies is a key factor in high protein solution viscosities. In the present work, a coarse-grained computational model accounting for electrostatic, dispersion, and long-ranged hydrodynamic interactions of two model monoclonal antibodies is applied to understand the nature of self-association, predicting the solution microstructure and resulting transport properties of the solution. For the proteins investigated, the structure factor across a range of solution conditions shows quantitative agreement with neutron-scattering experiments. We observe a homogeneous, dynamical association of the antibodies with no evidence of phase separation. Calculations of self-diffusivity and viscosity from coarse-grained dynamic simulations show the appropriate trends with concentration but, respectively, over- and under-predict the experimentally measured values. By adding constraints to the self-associated clusters that rigidify them under flow, prediction of the transport properties is significantly improved with respect to experimental measurements. We hypothesize that these rigidity constraints are associated with missing degrees of freedom in the coarse-grained model resulting from patchy and heterogeneous interactions among coarse-grained domains. These results demonstrate how structural anisotropy and anisotropy of interactions generated by features at the 2–5 nm length scale in antibodies are sufficient to recover the dynamics and rheological properties of these important macromolecular solutions.
中文翻译:

单克隆抗体溶液的结构和松弛
治疗性抗体的可逆自缔合是高蛋白溶液粘度的关键因素。在当前的工作中,考虑了两种模型单克隆抗体的静电,分散和远距离流体动力学相互作用的粗粒度计算模型被用于理解自缔合的性质,预测溶液的微观结构和所得溶液的传输性质。 。对于所研究的蛋白质,溶液条件范围内的结构因子与中子散射实验显示出定量的一致性。我们观察到抗体的均匀,动态关联,没有相分离的迹象。根据粗粒度动态模拟计算的自扩散系数和黏度显示出适当的浓度趋势,但是,高估和低估了实验测量值。通过对自相关簇增加约束以使其在流动下变硬,相对于实验测量,运输性质的预测得到了显着改善。我们假设这些刚性约束与由于粗粒度域之间的斑驳和异质相互作用而导致的粗粒度模型中缺少的自由度相关。这些结果表明,抗体中2–5 nm长度尺度上的特征所产生的结构各向异性和相互作用的各向异性如何足以恢复这些重要大分子溶液的动力学和流变特性。相对于实验测量,运输性能的预测得到了显着改善。我们假设这些刚性约束与由于粗粒度域之间的斑驳和异质相互作用而导致的粗粒度模型中缺少的自由度相关。这些结果表明,抗体中2–5 nm长度尺度上的特征所产生的结构各向异性和相互作用的各向异性如何足以恢复这些重要大分子溶液的动力学和流变特性。相对于实验测量,运输性能的预测得到了显着改善。我们假设这些刚性约束与由于粗粒度域之间的斑驳和异质相互作用而导致的粗粒度模型中缺少的自由度相关。这些结果表明,抗体中2–5 nm长度尺度上的特征所产生的结构各向异性和相互作用的各向异性如何足以恢复这些重要大分子溶液的动力学和流变特性。我们假设这些刚性约束与由于粗粒度域之间的斑驳和异质相互作用而导致的粗粒度模型中缺少的自由度相关。这些结果表明,抗体中2–5 nm长度尺度上的特征所产生的结构各向异性和相互作用的各向异性如何足以恢复这些重要大分子溶液的动力学和流变特性。我们假设这些刚性约束与由于粗粒度域之间的斑驳和异质相互作用而导致的粗粒度模型中缺少的自由度相关。这些结果表明,抗体中2–5 nm长度尺度上的特征所产生的结构各向异性和相互作用的各向异性如何足以恢复这些重要大分子溶液的动力学和流变特性。
更新日期:2018-02-22
中文翻译:

单克隆抗体溶液的结构和松弛
治疗性抗体的可逆自缔合是高蛋白溶液粘度的关键因素。在当前的工作中,考虑了两种模型单克隆抗体的静电,分散和远距离流体动力学相互作用的粗粒度计算模型被用于理解自缔合的性质,预测溶液的微观结构和所得溶液的传输性质。 。对于所研究的蛋白质,溶液条件范围内的结构因子与中子散射实验显示出定量的一致性。我们观察到抗体的均匀,动态关联,没有相分离的迹象。根据粗粒度动态模拟计算的自扩散系数和黏度显示出适当的浓度趋势,但是,高估和低估了实验测量值。通过对自相关簇增加约束以使其在流动下变硬,相对于实验测量,运输性质的预测得到了显着改善。我们假设这些刚性约束与由于粗粒度域之间的斑驳和异质相互作用而导致的粗粒度模型中缺少的自由度相关。这些结果表明,抗体中2–5 nm长度尺度上的特征所产生的结构各向异性和相互作用的各向异性如何足以恢复这些重要大分子溶液的动力学和流变特性。相对于实验测量,运输性能的预测得到了显着改善。我们假设这些刚性约束与由于粗粒度域之间的斑驳和异质相互作用而导致的粗粒度模型中缺少的自由度相关。这些结果表明,抗体中2–5 nm长度尺度上的特征所产生的结构各向异性和相互作用的各向异性如何足以恢复这些重要大分子溶液的动力学和流变特性。相对于实验测量,运输性能的预测得到了显着改善。我们假设这些刚性约束与由于粗粒度域之间的斑驳和异质相互作用而导致的粗粒度模型中缺少的自由度相关。这些结果表明,抗体中2–5 nm长度尺度上的特征所产生的结构各向异性和相互作用的各向异性如何足以恢复这些重要大分子溶液的动力学和流变特性。我们假设这些刚性约束与由于粗粒度域之间的斑驳和异质相互作用而导致的粗粒度模型中缺少的自由度相关。这些结果表明,抗体中2–5 nm长度尺度上的特征所产生的结构各向异性和相互作用的各向异性如何足以恢复这些重要大分子溶液的动力学和流变特性。我们假设这些刚性约束与由于粗粒度域之间的斑驳和异质相互作用而导致的粗粒度模型中缺少的自由度相关。这些结果表明,抗体中2–5 nm长度尺度上的特征所产生的结构各向异性和相互作用的各向异性如何足以恢复这些重要大分子溶液的动力学和流变特性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号