当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Equilibria in the Aqueous Ternary System (NH4)2SO4 + Na2SO4 + H2O at T = (303.15 and 313.15) K and p = 0.1 MPa
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-02-22 00:00:00 , DOI: 10.1021/acs.jced.7b00847 Dongchan Li 1, 2 , Rong Fan 1 , Xiaofu Guo 1, 2 , Sennan Yang 1 , Ziyi Zhang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-02-22 00:00:00 , DOI: 10.1021/acs.jced.7b00847 Dongchan Li 1, 2 , Rong Fan 1 , Xiaofu Guo 1, 2 , Sennan Yang 1 , Ziyi Zhang 1
Affiliation
|
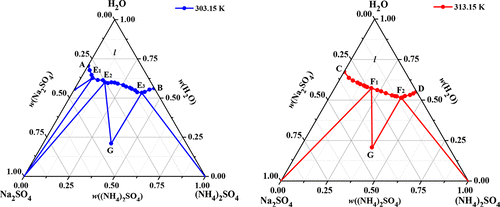
The solubility for the aqueous ternary system (NH4)2SO4 + Na2SO4 + H2O at T = (303.15 and 313.15) K and p = 0.1 MPa was investigated experimentally with the method of isothermal dissolution equilibrium. In the phase diagram of the ternary system at 303.15 K, there are eight crystallization regions, four univariant curves, and three invariant points corresponding to (Na2SO4·10H2O + Na2SO4), (Na2SO4 + Na2SO4·(NH4)2SO4·4H2O), and (Na2SO4·(NH4)2SO4·4H2O + (NH4)2SO4). However, in the phase diagram of the same ternary system at 313.15 K, the crystallization region of Na2SO4·10H2O is disappeared, and there are three single salt crystallization regions corresponding to Na2SO4, Na2SO4·(NH4)2SO4·4H2O, and (NH4)2SO4. A comparison of the phase diagrams for this ternary system at (273.15, 288.15, 298.15, 303.15, 313.15, and 333.15) K shows that the area of Na2SO4·(NH4)2SO4·4H2O and (NH4)2SO4 is increased obviously, whereas the area of Na2SO4 (or Na2SO4·10H2O) is decreased significantly and no solid solution was found. The experimental phase equilibria and phase diagrams can provide a fundamental basis for salt recovery in industrial wastewaters.
中文翻译:

三元水溶液(NH 4)2 SO 4 + Na 2 SO 4 + H 2 O在T =(303.15和313.15)K和p = 0.1 MPa时的相平衡
采用等温溶解平衡法,实验研究了三元体系(NH 4)2 SO 4 + Na 2 SO 4 + H 2 O在T =(303.15和313.15)K和p = 0.1 MPa时的溶解度。在303.15 K处的三元体系相图中,有八个结晶区,四个单变量曲线和三个对应于(Na 2 SO 4 ·10H 2 O + Na 2 SO 4),(Na 2 SO 4 + Na 2 SO 4·(NH 4)2 SO 4 ·4H 2 O)和(Na 2 SO 4 ·(NH 4)2 SO 4 ·4H 2 O +(NH 4)2 SO 4)。然而,在同一三元体系的相图中,在313.15 K处,Na 2 SO 4 ·10H 2 O的结晶区域消失了,并且存在对应于Na 2 SO 4,Na 2 SO 4 ·的三个单盐结晶区域。 (NH 4)2SO 4 ·4H 2 O和(NH 4)2 SO 4。比较该三元体系在(273.15、288.15、298.15、303.15、313.15和333.15)K时的相图,可以看出Na 2 SO 4 ·(NH 4)2 SO 4 ·4H 2 O和(NH 4)2 SO 4明显增加,而Na 2 SO 4(或Na 2 SO 4 ·10H 2)的面积增加O)显着降低,未发现固溶体。实验相平衡和相图可以为工业废水中的盐回收提供基础。
更新日期:2018-02-22
中文翻译:

三元水溶液(NH 4)2 SO 4 + Na 2 SO 4 + H 2 O在T =(303.15和313.15)K和p = 0.1 MPa时的相平衡
采用等温溶解平衡法,实验研究了三元体系(NH 4)2 SO 4 + Na 2 SO 4 + H 2 O在T =(303.15和313.15)K和p = 0.1 MPa时的溶解度。在303.15 K处的三元体系相图中,有八个结晶区,四个单变量曲线和三个对应于(Na 2 SO 4 ·10H 2 O + Na 2 SO 4),(Na 2 SO 4 + Na 2 SO 4·(NH 4)2 SO 4 ·4H 2 O)和(Na 2 SO 4 ·(NH 4)2 SO 4 ·4H 2 O +(NH 4)2 SO 4)。然而,在同一三元体系的相图中,在313.15 K处,Na 2 SO 4 ·10H 2 O的结晶区域消失了,并且存在对应于Na 2 SO 4,Na 2 SO 4 ·的三个单盐结晶区域。 (NH 4)2SO 4 ·4H 2 O和(NH 4)2 SO 4。比较该三元体系在(273.15、288.15、298.15、303.15、313.15和333.15)K时的相图,可以看出Na 2 SO 4 ·(NH 4)2 SO 4 ·4H 2 O和(NH 4)2 SO 4明显增加,而Na 2 SO 4(或Na 2 SO 4 ·10H 2)的面积增加O)显着降低,未发现固溶体。实验相平衡和相图可以为工业废水中的盐回收提供基础。










































 京公网安备 11010802027423号
京公网安备 11010802027423号