Ecotoxicology and Environmental Safety ( IF 6.2 ) Pub Date : 2018-02-22 , DOI: 10.1016/j.ecoenv.2018.02.013 Wenjing Shi , Changwei Lü , Jiang He , He En , Manshu Gao , Boyi Zhao , Bin Zhou , Haijun Zhou , Hualin Liu , Yu Zhang
|
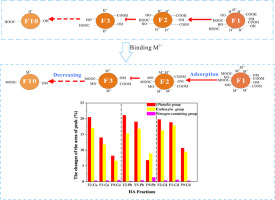
The composition and structure of Humic acid (HA) is so heterogeneous that it brings significant barriers to investigate the interaction between HA and heavy metal ions. The isolation of HA with relatively homogeneity is a key to reveal the binding mechanisms between HA and heavy metals. In this work, ten HA fractions (HAs) were obtained by sequential alkali extraction procedure and nature differences of the extracted HAs were considered as explanatory factors for binding characteristics of Cu2+, Pb2+ and Cd2+. The results indicate that more large molecular weight (MW) HA subunits, less carboxyl and phenolic group contents, weaker aromaticity and polarity were measured with increasing extractions, inducing weaker binding capacity of HAs. Ligand binding and bi-Langmuir models indicated that the sorption capacity and binding affinity of earlier extracted HAs were higher than the latter ones. The peak area changes at 3427, 1599, and 619 cm−1 pre- and post-adsorption in FTIR spectra suggested carboxyl, phenolic and nitrogen-containing groups were involved in the adsorption process. At the same time, the peak area difference between HAs and HAs-metal (ΔS) of phenolic groups were 8.22–20.50, 6.81–21.11 and 10.66–19.80% for Cu2+, Pb2+ and Cd2+, respectively, ΔS of carboxyl groups 6.64–17.03, 8.96–16.82 and 9.45–17.85% for Cu2+, Pb2+ and Cd2+, respectively, ΔS of nitrogen-containing groups 0.33–0.48, 0.20–1.38 and 0.31–0.59% for Cu2+, Pb2+ and Cd2+, respectively. ΔS of phenolic and carboxyl groups were larger than those of nitrogen-containing groups, implying that these two groups were the predominant binding sites suppliers for metal ions, which were also supported by the results of correlation analysis. This work is helpful to insight the environmental impacts of natural organic matter and the fate of heavy metals in natural environment.
中文翻译:

提取序列引起的腐殖酸组分的自然差异是重金属结合特性的解释性因素
腐殖酸(HA)的组成和结构是如此的异质,以至于在研究HA和重金属离子之间的相互作用时遇到了重大障碍。具有相对同质性的HA的分离是揭示HA与重金属之间结合机制的关键。在这项工作中,通过顺序碱提取程序获得了十个HA馏分(HAs),并且所提取的HAs的性质差异被认为是Cu 2 +,Pb 2+和Cd 2+结合特性的解释性因素。。结果表明,随着萃取次数的增加,分子量更大的HA亚基,羧基和酚基含量减少,芳香性和极性降低,导致HA的结合能力减弱。配体结合和bi-Langmuir模型表明,较早提取的HAs的吸附能力和结合亲和力高于后者。FTIR光谱在吸附前后分别在3427、1599和619 cm -1处出现峰面积变化,表明羧基,酚基和含氮基团参与了吸附过程。同时,Cu 2 +,Pb 2+和Cd 2+的酚基团的HAs和HAs金属之间的峰面积差(ΔS)为8.22–20.50、6.81–21.11和10.66–19.80%。对于Cu 2 +,Pb 2+和Cd 2+,羧基的ΔS分别为6.64-17.03、8.96-16.82和9.45-17.85%,含氮基团的ΔS分别为0.33-0.48、0.20-1.38和0.31- Cu 2 +,Pb 2+和Cd 2+分别为0.59%。酚基和羧基的ΔS大于含氮基团的ΔS,这表明这两个基团是金属离子的主要结合位点供应商,相关分析的结果也支持了这一点。这项工作有助于了解天然有机物对环境的影响以及自然环境中重金属的命运。











































 京公网安备 11010802027423号
京公网安备 11010802027423号