当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms ruling the partition of solutes in ionic-liquid-based aqueous biphasic systems – the multiple effects of ionic liquids†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-02-22 00:00:00 , DOI: 10.1039/c8cp00383a Helena Passos 1 , Teresa B V Dinis , Emanuel V Capela , Maria V Quental , Joana Gomes , Judite Resende , Pedro P Madeira , Mara G Freire , João A P Coutinho
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-02-22 00:00:00 , DOI: 10.1039/c8cp00383a Helena Passos 1 , Teresa B V Dinis , Emanuel V Capela , Maria V Quental , Joana Gomes , Judite Resende , Pedro P Madeira , Mara G Freire , João A P Coutinho
Affiliation
|
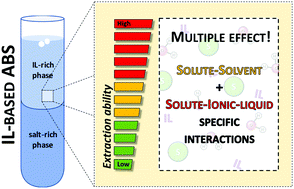
In the past decade, the remarkable potential of ionic-liquid-based aqueous biphasic systems (IL-based ABSs) to extract and purify a large range of valued-added biocompounds has been demonstrated. However, the translation of lab-scale experiments to an industrial scale has been precluded by a poor understanding of the molecular-level mechanisms ruling the separation or partition of target compounds between the coexisting phases. To overcome this limitation, we carried out a systematic evaluation of specific interactions, induced by ILs and several salts used as phase-forming components, and their impact on the partition of several solutes in IL-based ABSs. To this end, the physicochemical characterization of ABSs composed of imidazolium-based ILs, three salts (Na2SO4, K2CO3 and K3C6H5O7) and water was performed. The ability of the coexisting phases to participate in different solute–solvent interactions (where “solvent” corresponds to each ABS phase) was estimated based on the Gibbs free energy of transfer of a methylene group between the phases in equilibrium, ΔG(CH2), and on the Kamlet–Taft parameters – dipolarity/polarizability (π*), hydrogen-bonding donor acidity (α) and hydrogen-bonding acceptor basicity (β) – of the coexisting phases. Relationships between the partition coefficients, the phase properties expressed as Kamlet–Taft parameters and COSMO-RS descriptors were established, highlighting the ability of ILs to establish specific interactions with given solutes. The assembled results clearly support the idea that the partition of solutes in IL-based ABSs is due to multiple effects resulting from both global solute–solvent and specific solute–IL interactions. Solute–IL specific interactions are often dominant in IL-based ABSs, explaining the higher partition coefficients, extraction efficiencies and selectivities observed with these systems when compared to more traditional ones majorly composed of polymers.
中文翻译:

离子液体水性双相系统中溶质分配的决定机制——离子液体的多重效应†
在过去的十年中,基于离子液体的水性双相系统(基于 IL 的 ABS)在提取和纯化大量增值生物化合物方面的巨大潜力已得到证明。然而,由于对控制共存相之间目标化合物的分离或分配的分子水平机制了解甚少,实验室规模的实验无法转化为工业规模。为了克服这一限制,我们对 IL 和用作相形成组分的几种盐诱导的特定相互作用及其对基于 IL 的 ABS 中几种溶质分配的影响进行了系统评估。为此,对由咪唑基离子液体、三种盐(Na 2 SO 4、K 2 CO 3和K 3 C 6 H 5 O 7)和水组成的ABS进行了物理化学表征。共存相参与不同溶质-溶剂相互作用的能力(其中“溶剂”对应于每个 ABS 相)是根据平衡相之间亚甲基转移的吉布斯自由能 Δ G ( CH 2 ),以及共存相的 Kamlet-Taft 参数——偶极性/极化性 (π*)、氢键供体酸性 ( α ) 和氢键受体碱性 ( β )。建立了分配系数、以 Kamlet-Taft 参数表示的相特性和 COSMO-RS 描述符之间的关系,强调了离子液体与给定溶质建立特定相互作用的能力。汇总的结果清楚地支持这样的观点,即基于 IL 的 ABS 中溶质的分配是由于整体溶质 - 溶剂和特定溶质 - IL 相互作用产生的多重效应所致。溶质-IL 特异性相互作用通常在基于 IL 的 ABS 中占主导地位,这解释了与主要由聚合物组成的更传统的系统相比,这些系统观察到的更高的分配系数、萃取效率和选择性。
更新日期:2018-02-22
中文翻译:

离子液体水性双相系统中溶质分配的决定机制——离子液体的多重效应†
在过去的十年中,基于离子液体的水性双相系统(基于 IL 的 ABS)在提取和纯化大量增值生物化合物方面的巨大潜力已得到证明。然而,由于对控制共存相之间目标化合物的分离或分配的分子水平机制了解甚少,实验室规模的实验无法转化为工业规模。为了克服这一限制,我们对 IL 和用作相形成组分的几种盐诱导的特定相互作用及其对基于 IL 的 ABS 中几种溶质分配的影响进行了系统评估。为此,对由咪唑基离子液体、三种盐(Na 2 SO 4、K 2 CO 3和K 3 C 6 H 5 O 7)和水组成的ABS进行了物理化学表征。共存相参与不同溶质-溶剂相互作用的能力(其中“溶剂”对应于每个 ABS 相)是根据平衡相之间亚甲基转移的吉布斯自由能 Δ G ( CH 2 ),以及共存相的 Kamlet-Taft 参数——偶极性/极化性 (π*)、氢键供体酸性 ( α ) 和氢键受体碱性 ( β )。建立了分配系数、以 Kamlet-Taft 参数表示的相特性和 COSMO-RS 描述符之间的关系,强调了离子液体与给定溶质建立特定相互作用的能力。汇总的结果清楚地支持这样的观点,即基于 IL 的 ABS 中溶质的分配是由于整体溶质 - 溶剂和特定溶质 - IL 相互作用产生的多重效应所致。溶质-IL 特异性相互作用通常在基于 IL 的 ABS 中占主导地位,这解释了与主要由聚合物组成的更传统的系统相比,这些系统观察到的更高的分配系数、萃取效率和选择性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号