当前位置:
X-MOL 学术
›
Small Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Selection Mechanism of the Polymer Wrapping Technique toward Semiconducting Carbon Nanotubes
Small Methods ( IF 10.7 ) Pub Date : 2018-02-22 , DOI: 10.1002/smtd.201700335 Jorge Mario Salazar-Rios 1 , Wytse Talsma 1 , Vladimir Derenskyi 1 , Widianta Gomulya 1, 2 , Tina Keller 3 , Martin Fritsch 3 , Sebastian Kowalski 3 , Eduard Preis 3 , Ming Wang 4 , Sybille Allard 3 , Guillermo Carlos Bazan 4 , Ullrich Scherf 3 , Maria Cristina dos Santos 1 , Maria Antonietta Loi 1
Small Methods ( IF 10.7 ) Pub Date : 2018-02-22 , DOI: 10.1002/smtd.201700335 Jorge Mario Salazar-Rios 1 , Wytse Talsma 1 , Vladimir Derenskyi 1 , Widianta Gomulya 1, 2 , Tina Keller 3 , Martin Fritsch 3 , Sebastian Kowalski 3 , Eduard Preis 3 , Ming Wang 4 , Sybille Allard 3 , Guillermo Carlos Bazan 4 , Ullrich Scherf 3 , Maria Cristina dos Santos 1 , Maria Antonietta Loi 1
Affiliation
|
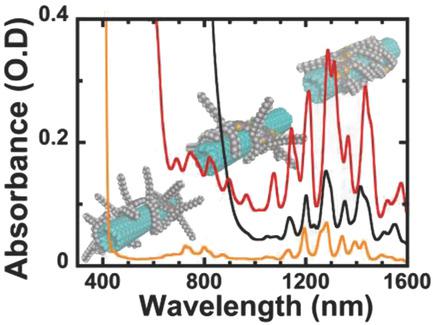
Noncovalent functionalization of single‐walled carbon nanotubes (SWNTs) using π‐conjugated polymers has become one of the most effective techniques to select semiconducting SWNTs (s‐SWNTs). Several conjugated polymers are used, but their ability to sort metallic and semiconducting species, as well as the dispersions yields, varies as a function of their chemical structure. Here, three polymers are compared, namely, poly[2,6‐(4,4‐bis‐(2‐dodecyl)‐4H‐cyclopenta[2,1‐b;3,4b′]dithiophene)‐alt‐4,7(2,1,3‐benzothiadiazole)] (P12CPDTBT), poly(9,9‐di‐n‐dodecylfluorenyl‐2,7‐diyl) (PF12), and poly(3‐dodecylthiophene‐2,5‐diyl) (P3DDT) in their ability to select two types of carbon nanotubes comprising small (≈1 nm) and large (≈1.5 nm) diameters. P12CPDTBT is a better dispersant than PF12 for small diameter nanotubes, while both polymers are good dispersants of large diameter nanotubes. However, these dispersions contain metallic species. P3DDT, instead presents the best overall performance regarding the selectivity toward semiconducting species, with a dispersion yield for s‐SWNTs of 15% for small and 21% for large diameter nanotubes. These results are rationalized in terms of electronic and chemical structure showing that: (i) the binding energy is stronger when more alkyl lateral chains adsorb on the nanotube surface; (ii) the binding energy is stronger when the polymer backbone is more flexible; (iii) the purity of the dispersions seems to depend on a strong polymer–nanotube interaction.
中文翻译:

了解聚合物包裹技术对半导体碳纳米管的选择机理
使用π共轭聚合物对单壁碳纳米管(SWNT)进行非共价官能化已成为选择半导体SWNT(s-SWNT)的最有效技术之一。使用了几种共轭聚合物,但是它们对金属和半导体种类进行分选的能力以及分散体的收率随其化学结构的变化而变化。在这里,比较了三种聚合物,即聚[2,6-(4,4-双-(2-十二烷基)-4H-环戊[2,1-b; 3,4b']二噻吩)-alt-4, 7(2,1,3-苯并噻二唑)](P12CPDTBT),聚(9,9-二-正十二烷基芴基-2,7-二基)(PF12)和聚(3-十二烷基噻吩-2,5-二基) (P3DDT)在选择包括小直径(≈1nm)和大直径(≈1.5nm)的两种类型的碳纳米管的能力。对于小直径纳米管,P12CPDTBT是比PF12更好的分散剂,而两种聚合物都是大直径纳米管的良好分散剂。但是,这些分散体包含金属物质。相反,P3DDT在针对半导体物质的选择性方面表现出最佳的整体性能,对于小直径纳米管,s-SWNTs的分散产率为15%,而大直径纳米管的分散产率为21%。这些结果在电子和化学结构方面是合理的,表明:(i)当更多的烷基侧链吸附在纳米管表面时,结合能更强;(ii)当聚合物主链更柔韧时,结合能更强;(iii)分散液的纯度似乎取决于强烈的聚合物-纳米管相互作用。取而代之的是在针对半导体种类的选择性方面表现出最佳的整体性能,对于小直径的s-SWNTs,分散直径为15%,对于大直径的纳米管,其分散率为21%。这些结果在电子和化学结构方面是合理的,表明:(i)当更多的烷基侧链吸附在纳米管表面时,结合能更强;(ii)当聚合物主链更柔韧时,结合能更强;(iii)分散液的纯度似乎取决于强烈的聚合物-纳米管相互作用。取而代之的是在针对半导体种类的选择性方面表现出最佳的整体性能,对于小直径的s-SWNTs,分散直径为15%,对于大直径的纳米管,其分散率为21%。这些结果在电子和化学结构方面是合理的,表明:(i)当更多的烷基侧链吸附在纳米管表面时,结合能更强;(ii)当聚合物主链更柔韧时,结合能更强;(iii)分散液的纯度似乎取决于强烈的聚合物-纳米管相互作用。(ii)当聚合物主链更柔韧时,结合能更强;(iii)分散液的纯度似乎取决于强烈的聚合物-纳米管相互作用。(ii)当聚合物主链更柔韧性时,结合能更强;(iii)分散液的纯度似乎取决于强烈的聚合物-纳米管相互作用。
更新日期:2018-02-22
中文翻译:

了解聚合物包裹技术对半导体碳纳米管的选择机理
使用π共轭聚合物对单壁碳纳米管(SWNT)进行非共价官能化已成为选择半导体SWNT(s-SWNT)的最有效技术之一。使用了几种共轭聚合物,但是它们对金属和半导体种类进行分选的能力以及分散体的收率随其化学结构的变化而变化。在这里,比较了三种聚合物,即聚[2,6-(4,4-双-(2-十二烷基)-4H-环戊[2,1-b; 3,4b']二噻吩)-alt-4, 7(2,1,3-苯并噻二唑)](P12CPDTBT),聚(9,9-二-正十二烷基芴基-2,7-二基)(PF12)和聚(3-十二烷基噻吩-2,5-二基) (P3DDT)在选择包括小直径(≈1nm)和大直径(≈1.5nm)的两种类型的碳纳米管的能力。对于小直径纳米管,P12CPDTBT是比PF12更好的分散剂,而两种聚合物都是大直径纳米管的良好分散剂。但是,这些分散体包含金属物质。相反,P3DDT在针对半导体物质的选择性方面表现出最佳的整体性能,对于小直径纳米管,s-SWNTs的分散产率为15%,而大直径纳米管的分散产率为21%。这些结果在电子和化学结构方面是合理的,表明:(i)当更多的烷基侧链吸附在纳米管表面时,结合能更强;(ii)当聚合物主链更柔韧时,结合能更强;(iii)分散液的纯度似乎取决于强烈的聚合物-纳米管相互作用。取而代之的是在针对半导体种类的选择性方面表现出最佳的整体性能,对于小直径的s-SWNTs,分散直径为15%,对于大直径的纳米管,其分散率为21%。这些结果在电子和化学结构方面是合理的,表明:(i)当更多的烷基侧链吸附在纳米管表面时,结合能更强;(ii)当聚合物主链更柔韧时,结合能更强;(iii)分散液的纯度似乎取决于强烈的聚合物-纳米管相互作用。取而代之的是在针对半导体种类的选择性方面表现出最佳的整体性能,对于小直径的s-SWNTs,分散直径为15%,对于大直径的纳米管,其分散率为21%。这些结果在电子和化学结构方面是合理的,表明:(i)当更多的烷基侧链吸附在纳米管表面时,结合能更强;(ii)当聚合物主链更柔韧时,结合能更强;(iii)分散液的纯度似乎取决于强烈的聚合物-纳米管相互作用。(ii)当聚合物主链更柔韧时,结合能更强;(iii)分散液的纯度似乎取决于强烈的聚合物-纳米管相互作用。(ii)当聚合物主链更柔韧性时,结合能更强;(iii)分散液的纯度似乎取决于强烈的聚合物-纳米管相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号