Chem ( IF 19.1 ) Pub Date : 2018-02-22 , DOI: 10.1016/j.chempr.2018.01.019 Yuhan Peng , Liangbing Wang , Qiquan Luo , Yun Cao , Yizhou Dai , Zhongling Li , Hongliang Li , Xusheng Zheng , Wensheng Yan , Jinlong Yang , Jie Zeng
|
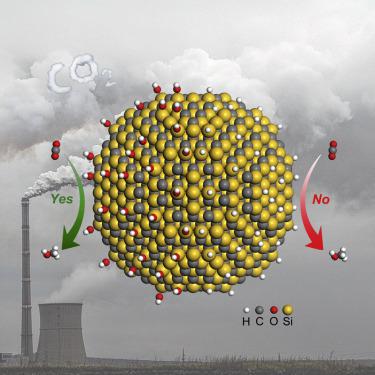
Exploring how hydrophilicity regulates catalytic properties at the molecular level remains a grand challenge, although it has great potential to offer guidelines for developing highly efficient catalysts and deepen the mechanistic understanding of heterogeneous catalysis. Here, we provide molecular-level insight into the influence of surface hydroxyl groups on hydrophilic SiC quantum dots (QDs) on CO2 hydrogenation. In CO2 hydrogenation into methanol, SiC QDs exhibited higher catalytic activity and lower activation energy than commercial SiC. Mechanistic studies revealed that the surface hydroxyl species on SiC QDs was directly involved in CO2 hydrogenation through the addition of H atoms in hydroxyl groups into CO2 to form HCOO* as the intermediate. The unique reaction path decreased the energy barrier for the formation of HCOO*, facilitating the activation of CO2. Our understanding of surface hydrophilicity directly instructs the development of efficient catalysts toward CO2 hydrogenation.
中文翻译:

分子水平的洞察力,以了解羟基如何提高CO 2加氢制甲醇中的催化活性
探索亲水性如何在分子水平上调节催化性能仍然是一个巨大的挑战,尽管它具有很大的潜力,可为开发高效催化剂提供指导,并加深对多相催化的机理的理解。在这里,我们提供了分子水平的洞察力,以了解表面羟基对亲水性SiC量子点(QDs)对CO 2加氢的影响。在将CO 2加氢成甲醇的过程中,SiC量子点比商用SiC表现出更高的催化活性和更低的活化能。机理研究表明,SiC量子点表面的羟基物种通过将羟基中的H原子添加到CO 2中而直接参与了CO 2加氢。形成HCOO *作为中间体。独特的反应路径减少了形成HCOO *的能垒,促进了CO 2的活化。我们对表面亲水性的理解直接指导了向CO 2加氢的高效催化剂的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号