Chem ( IF 19.1 ) Pub Date : 2018-02-22 , DOI: 10.1016/j.chempr.2018.01.017 Kun Zhao , Longhui Duan , Shibo Xu , Julong Jiang , Yao Fu , Zhenhua Gu
|
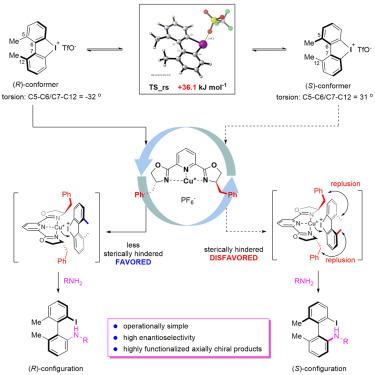
Atropisomers are stereoisomers arising from the restricted rotation around a single bond. In particular, biaryl atropisomers represent an important class of compounds, as they are widely present in natural products, ligands and pharmaceutical molecules. However, the preparation of structurally diverse biaryl atropisomers under mild conditions is a significant challenge. Here, we describe a Cu-bis(oxazolinyl)pyridine-catalyzed asymmetric ring-opening amination reaction of cyclic diaryliodoniums. Increasing the torsional strain of these cyclic compounds significantly improved the reactivity of cyclic diaryliodoniums. Computational investigation indicated that the two conformers of the cyclic diaryliodoniums had a low rotational barrier, and generally the reaction achieved high yields and high enantioselectivity (up to >99% ee). Furthermore, this ring-opening amination reaction also featured high atom economy in comparison with traditional reactions involving diaryliodonium. Finally, we propose a catalytic cycle and a mechanistic model that accounts for the observed enantioselectivity.
中文翻译:

铜催化的对映选择性开环反应中环二芳基铵的扭转应变增强的反应性
阻转异构体是由于绕单键旋转受限而产生的立体异构体。特别地,联芳基阻转异构体代表一类重要的化合物,因为它们广泛存在于天然产物,配体和药物分子中。然而,在温和条件下制备结构多样的联芳基阻转异构体是一项重大挑战。在这里,我们描述了一个铜-双(恶唑啉基)吡啶催化的环状二芳基碘鎓的不对称开环胺化反应。增加这些环状化合物的扭转应变可显着提高环状二芳基碘鎓的反应性。计算研究表明,环状二芳基碘鎓的两个构象异构体具有较低的旋转势垒,并且通常该反应实现了高收率和高对映选择性(高达> 99%ee)。此外,与涉及二芳基碘鎓的传统反应相比,这种开环胺化反应还具有较高的原子经济性。最后,我们提出了一个催化循环和一个机制模型,用于解释所观察到的对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号