当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification and Structure-Guided Development of Pyrimidinone Based USP7 Inhibitors
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00512 Colin R. O’Dowd 1 , Matthew D. Helm 1 , J. S. Shane Rountree 1 , Jakub T. Flasz 2 , Elias Arkoudis 2 , Hugues Miel 1 , Peter R. Hewitt 1 , Linda Jordan 1 , Oliver Barker 1 , Caroline Hughes 1 , Ewelina Rozycka 1 , Eamon Cassidy 1 , Keeva McClelland 1 , Ewa Odrzywol 1 , Natalie Page 1 , Stephanie Feutren-Burton 1 , Scarlett Dvorkin 2 , Gerald Gavory 1 , Timothy Harrison 1, 2
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00512 Colin R. O’Dowd 1 , Matthew D. Helm 1 , J. S. Shane Rountree 1 , Jakub T. Flasz 2 , Elias Arkoudis 2 , Hugues Miel 1 , Peter R. Hewitt 1 , Linda Jordan 1 , Oliver Barker 1 , Caroline Hughes 1 , Ewelina Rozycka 1 , Eamon Cassidy 1 , Keeva McClelland 1 , Ewa Odrzywol 1 , Natalie Page 1 , Stephanie Feutren-Burton 1 , Scarlett Dvorkin 2 , Gerald Gavory 1 , Timothy Harrison 1, 2
Affiliation
|
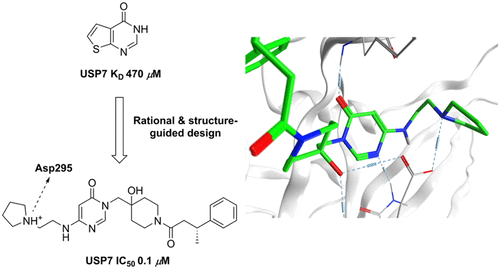
Ubiquitin specific protease 7 (USP7, HAUSP) has become an attractive target in drug discovery due to the role it plays in modulating Mdm2 levels and consequently p53. Increasing interest in USP7 is emerging due to its potential involvement in oncogenic pathways as well as possible roles in both metabolic and immune disorders in addition to viral infections. Potent, novel, and selective inhibitors of USP7 have been developed using both rational and structure-guided design enabled by high-resolution cocrystallography. Initial hits were identified via fragment-based screening, scaffold-hopping, and hybridization exercises. Two distinct subseries are described along with associated structure–activity relationship trends, as are initial efforts aimed at developing compounds suitable for in vivo experiments. Overall, these discoveries will enable further research into the wider biological role of USP7.
中文翻译:

基于嘧啶酮的USP7抑制剂的鉴定与结构指导
泛素特异性蛋白酶7(USP7,HAUSP)由于在调节Mdm2水平(进而调节p53)中的作用,已成为药物发现中的引人注目的靶标。由于USP7可能参与致癌途径以及除病毒感染外在代谢和免疫疾病中的可能作用,因此人们对USP7的兴趣也越来越高。USP7的强效,新型和选择性抑制剂已通过高分辨率共结晶技术的合理设计和结构指导设计得到开发。最初的命中是通过基于片段的筛选,支架跳跃和杂交练习来确定的。描述了两个不同的子系列以及相关的结构-活性关系趋势,以及旨在开发适用于体内化合物的初步努力实验。总体而言,这些发现将有助于进一步研究USP7的更广泛的生物学作用。
更新日期:2018-02-21
中文翻译:

基于嘧啶酮的USP7抑制剂的鉴定与结构指导
泛素特异性蛋白酶7(USP7,HAUSP)由于在调节Mdm2水平(进而调节p53)中的作用,已成为药物发现中的引人注目的靶标。由于USP7可能参与致癌途径以及除病毒感染外在代谢和免疫疾病中的可能作用,因此人们对USP7的兴趣也越来越高。USP7的强效,新型和选择性抑制剂已通过高分辨率共结晶技术的合理设计和结构指导设计得到开发。最初的命中是通过基于片段的筛选,支架跳跃和杂交练习来确定的。描述了两个不同的子系列以及相关的结构-活性关系趋势,以及旨在开发适用于体内化合物的初步努力实验。总体而言,这些发现将有助于进一步研究USP7的更广泛的生物学作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号