当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Weaker N-Terminal Interactions for the Protective over the Causative Aβ Peptide Dimer Mutants
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acschemneuro.7b00412 Bhanushee Sharma 1 , Srivathsan V. Ranganathan 2 , Georges Belfort 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acschemneuro.7b00412 Bhanushee Sharma 1 , Srivathsan V. Ranganathan 2 , Georges Belfort 1
Affiliation
|
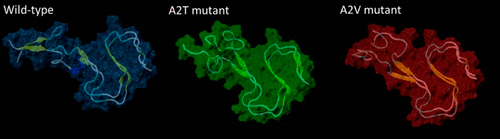
Knowing that abeta amyloid peptide (Aβ42) dimers are the smallest and most abundant neurotoxic oligomers for Alzheimer’s disease (AD), we used molecular simulations with advanced sampling methods (replica-exchange) to characterize and compare interactions between the N-termini (residues 1–16) of wild type (WT-WT) and five mutant dimers under constrained and unconstrained conditions. The number of contacts and distances between the N-termini, and contact maps of their conformational landscape illustrate substantial differences for a single residue change. The N-terminal contacts are significantly diminished for the dimers containing the monomers that protect against (WT-A2T) as compared with those that predispose toward (A2V-A2V) AD and for the control WT-WT dimers. The reduced number of N-terminal contacts not only occurs at or near the second residue mutations but also is distributed through to the 10th residue. These findings provide added support to the accumulating evidence for the “N-terminal hypothesis of AD” and offer an alternate mechanism for the cause of protection from the A2T mutant.
中文翻译:

较弱的N末端相互作用对Aβ肽二聚体突变体具有保护作用。
知道abeta淀粉样肽(Aβ42)二聚体是阿尔茨海默氏病(AD)最小,最丰富的神经毒性寡聚体,我们使用分子模拟和先进的采样方法(复制品交换)来表征和比较野生型N-末端(残基1–16)之间的相互作用( (WT-WT)和五个突变二聚体在受限和非受限条件下。N-末端之间的接触数和距离,以及其构象态势的接触图说明了单个残基变化的实质性差异。与倾向于保护(A2V-A2V)AD的单体相比,含有保护(WT-A2T)的单体的二聚体和对照WT-WT二聚体的N端触点明显减少。N末端接触数量的减少不仅在第二个残基突变处或附近发生,而且一直分布到第10个残基。这些发现为“ AD的N末端假说”的不断积累的证据提供了额外的支持,并为引起A2T突变体保护的原因提供了另一种机制。
更新日期:2018-02-21
中文翻译:

较弱的N末端相互作用对Aβ肽二聚体突变体具有保护作用。
知道abeta淀粉样肽(Aβ42)二聚体是阿尔茨海默氏病(AD)最小,最丰富的神经毒性寡聚体,我们使用分子模拟和先进的采样方法(复制品交换)来表征和比较野生型N-末端(残基1–16)之间的相互作用( (WT-WT)和五个突变二聚体在受限和非受限条件下。N-末端之间的接触数和距离,以及其构象态势的接触图说明了单个残基变化的实质性差异。与倾向于保护(A2V-A2V)AD的单体相比,含有保护(WT-A2T)的单体的二聚体和对照WT-WT二聚体的N端触点明显减少。N末端接触数量的减少不仅在第二个残基突变处或附近发生,而且一直分布到第10个残基。这些发现为“ AD的N末端假说”的不断积累的证据提供了额外的支持,并为引起A2T突变体保护的原因提供了另一种机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号