当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic Structure and Bonding Situation in M2O2 (M = Be, Mg, Ca) Rhombic Clusters
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acs.jpca.8b01335 Wan-Lu Li 1 , Jun-Bo Lu 1 , Lili Zhao 2 , Robert Ponec 3 , David L. Cooper 4 , Jun Li 1 , Gernot Frenking 2, 5
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acs.jpca.8b01335 Wan-Lu Li 1 , Jun-Bo Lu 1 , Lili Zhao 2 , Robert Ponec 3 , David L. Cooper 4 , Jun Li 1 , Gernot Frenking 2, 5
Affiliation
|
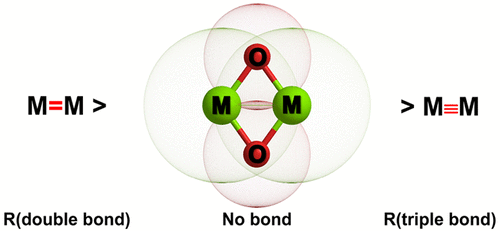
Quantum chemical calculations using ab initio methods at the CCSD(T) level and density functional theory have been carried out for the title molecules. The electronic structures of the molecules were analyzed with a variety of charge and energy decomposition methods. The equilibrium geometries of the M2O2 rhombic clusters exhibit very short distances between the transannular metal atoms M = Be, Mg, Ca. The calculated distances are close to standard values between double and triple bonds, but there are no chemical M–M bonds. The metal atoms M carry large positive partial charges, which are even bigger than in diatomic MO. The valence electrons of M are essentially shifted toward oxygen in M2O2, which makes it possible that there is practically no electronic charge in the region between the metal atoms. The bond dissociation energies for fragmentation of M2O2 into two metal oxides MO are very large. The metal–oxide bonds in the rhombic clusters are shorter and stronger than in diatomic MO. A detailed analysis of the electronic structure suggests that there is no significant direct M–M interaction in the M2O2 rhombic clusters, albeit weak three-center M–O–M bonding.
中文翻译:

M 2 O 2(M = Be,Mg,Ca)菱形簇中的电子结构和键合态
已经对标题分子使用了从头算方法在CCSD(T)级别上进行了量子化学计算,并利用密度泛函理论进行了计算。用各种电荷和能量分解方法分析了分子的电子结构。M 2 O 2菱形簇的平衡几何形状在跨环形金属原子M = Be,Mg,Ca之间显示出非常短的距离。计算的距离接近双键和三键之间的标准值,但是没有化学M–M键。金属原子M带有较大的正部分电荷,甚至比双原子MO中的电荷大。M的价电子基本上在M 2 O 2中向氧移动,这使得在金属原子之间的区域中实际上不存在电子电荷成为可能。用于将M 2 O 2分裂成两种金属氧化物MO的键解离能非常大。菱形团簇中的金属氧化物键比双原子MO短而强。对电子结构的详细分析表明,尽管三中心M–O–M键很弱,M 2 O 2菱形簇中也没有明显的直接M–M相互作用。
更新日期:2018-02-21
中文翻译:

M 2 O 2(M = Be,Mg,Ca)菱形簇中的电子结构和键合态
已经对标题分子使用了从头算方法在CCSD(T)级别上进行了量子化学计算,并利用密度泛函理论进行了计算。用各种电荷和能量分解方法分析了分子的电子结构。M 2 O 2菱形簇的平衡几何形状在跨环形金属原子M = Be,Mg,Ca之间显示出非常短的距离。计算的距离接近双键和三键之间的标准值,但是没有化学M–M键。金属原子M带有较大的正部分电荷,甚至比双原子MO中的电荷大。M的价电子基本上在M 2 O 2中向氧移动,这使得在金属原子之间的区域中实际上不存在电子电荷成为可能。用于将M 2 O 2分裂成两种金属氧化物MO的键解离能非常大。菱形团簇中的金属氧化物键比双原子MO短而强。对电子结构的详细分析表明,尽管三中心M–O–M键很弱,M 2 O 2菱形簇中也没有明显的直接M–M相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号