当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Release of Terminal Alkynes via Tandem Photodeprotection and Decarboxylation of o-Nitrobenzyl Arylpropiolates in a Flow Microchannel Reactor
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00812 Behabitu Ergette Tebikachew 1 , Karl Börjesson 2 , Nina Kann 1 , Kasper Moth-Poulsen 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00812 Behabitu Ergette Tebikachew 1 , Karl Börjesson 2 , Nina Kann 1 , Kasper Moth-Poulsen 1
Affiliation
|
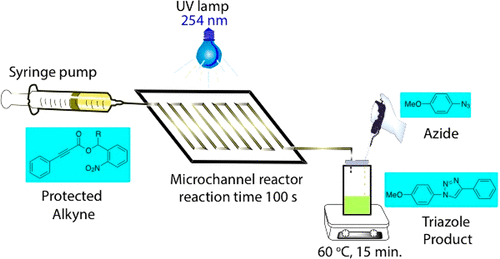
Photocleavable protecting groups (PPGs) offer a complementary protection paradigm compared to traditional protection groups. Herein, an o-nitrobenzyl (NB) PPG was employed to protect a variety of arylpropiolic acids. Upon a cascade of light-triggered photodeprotection in a microchannel reactor (residence times of 100–500 s), followed by Cu-catalyzed decarboxylation at 60 °C, the NB-protected arylpropiolic acid afforded a terminal alkyne. This terminal alkyne was further reacted in situ with an azide via click chemistry to yield a 1,2,3-triazole in a one-pot reaction. Furthermore, the effect of different substituents (methyl, vinyl, allyl, and phenyl) at the benzylic position on the rate of photodeprotection was studied. The quantum yields of photolysis for the benzylic-substituted esters were determined to be as high as 0.45 compared to the unsubstituted ester with a 0.08 quantum yield of photolysis.
中文翻译:

在流动微通道反应器中,通过串联光脱保护和邻硝基硝基苄基丙酸丙酯的串联光脱保护释放末端炔烃
与传统的保护基团相比,可光裂解的保护基团(PPG)提供了互补的保护范式。在本文中,使用邻硝基苄基(NB)PPG来保护各种芳基丙酸。在微通道反应器中级联触发光触发的光脱保护(停留时间为100-500 s),然后在60°C下进行Cu催化的脱羧反应,NB保护的芳基丙酸提供了末端炔。该末端炔烃在原位进一步反应一键反应通过叠氮化物与叠氮化物通过点击化学反应生成1,2,3-三唑。此外,研究了苄基位置上不同取代基(甲基,乙烯基,烯丙基和苯基)对光脱保护速率的影响。经测定,与未取代的酯相比,苄基取代的酯的光解量子产率高达0.45,光解的量子产率为0.08。
更新日期:2018-02-21
中文翻译:

在流动微通道反应器中,通过串联光脱保护和邻硝基硝基苄基丙酸丙酯的串联光脱保护释放末端炔烃
与传统的保护基团相比,可光裂解的保护基团(PPG)提供了互补的保护范式。在本文中,使用邻硝基苄基(NB)PPG来保护各种芳基丙酸。在微通道反应器中级联触发光触发的光脱保护(停留时间为100-500 s),然后在60°C下进行Cu催化的脱羧反应,NB保护的芳基丙酸提供了末端炔。该末端炔烃在原位进一步反应一键反应通过叠氮化物与叠氮化物通过点击化学反应生成1,2,3-三唑。此外,研究了苄基位置上不同取代基(甲基,乙烯基,烯丙基和苯基)对光脱保护速率的影响。经测定,与未取代的酯相比,苄基取代的酯的光解量子产率高达0.45,光解的量子产率为0.08。











































 京公网安备 11010802027423号
京公网安备 11010802027423号