当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nano-Assembly of Pamitoyl-Bioconjugated Coenzyme-A for Combinatorial Chemo-Biologics in Transcriptional Therapy
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00117 Santosh K. Misra 1, 2 , Taylor L. Kampert 1, 2 , Dipanjan Pan 1, 2
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00117 Santosh K. Misra 1, 2 , Taylor L. Kampert 1, 2 , Dipanjan Pan 1, 2
Affiliation
|
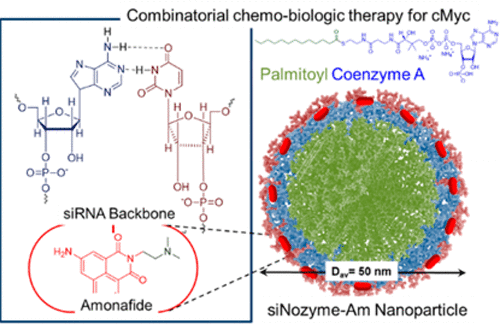
Pathogenesis, the biological mechanism that leads to the diseased state, of many cancers is driven by interruptions to the role of Myc oncoprotein, a regulator protein that codes for a transcription factor. One of the most significant biological interruptions to Myc protein is noted as its dimerization with Max protein, another important factor of family of transcription factors. Binding of this heterodimer to E-Boxes, enhancer boxes as DNA response element found in some eukaryotes that act as a protein-binding site and have been found to regulate gene expression, are interrupted to regulate cancer pathogenesis. The systemic effectiveness of potent small molecule inhibitors of Myc-Max dimerization has been limited by poor bioavailability, rapid metabolism, and inadequate target site penetration. The potential of gene therapy for targeting Myc can be fully realized by successful synthesis of a smart cargo. We developed a “nuclein” type nanoparticle “siNozyme” (45 ± 5 nm) from nanoassembly of pamitoyl-bioconjugated acetyl coenzyme-A for stable incorporation of chemotherapeutics and biologics to achieve remarkable growth inhibition of human melanoma. Results indicated that targeting transcriptional gene cMyc with siRNA with codelivery of a topoisomerase inhibitor, amonafide caused ∼90% growth inhibition and 95% protein inhibition.
中文翻译:

氨基甲酰基-生物共轭辅酶-A的组合,在转录治疗中的组合化学生物学家。
致病性是导致许多疾病致病状态的生物学机制,是由Myc癌蛋白(一种编码转录因子的调节蛋白)的作用中断引起的。Myc蛋白最重要的生物学干扰之一是其与Max蛋白(转录因子家族的另一重要因子)的二聚作用。这种异二聚体与E-Boxs的结合,即增强子盒,作为一些在真核生物中作为蛋白质结合位点并已发现调节基因表达的DNA响应元件,被中断以调节癌症的发病机理。强效的小分子Myc-Max二聚化抑制剂的全身有效性受到不良的生物利用度,快速的新陈代谢和不足的目标位点渗透的限制。通过成功合成智能货物,可以完全实现靶向Myc的基因疗法的潜力。我们从氨基甲酰基-生物共轭的乙酰辅酶-A的纳米组装中开发了一种“核素”型纳米颗粒“ siNozyme”(45±5 nm),用于稳定结合化学疗法和生物制剂以实现对人类黑素瘤的显着抑制。结果表明,以拓扑异构酶抑制剂,阿莫那肽的代码传递作用与siRNA靶向转录基因cMyc导致约90%的生长抑制和95%的蛋白质抑制。
更新日期:2018-02-21
中文翻译:

氨基甲酰基-生物共轭辅酶-A的组合,在转录治疗中的组合化学生物学家。
致病性是导致许多疾病致病状态的生物学机制,是由Myc癌蛋白(一种编码转录因子的调节蛋白)的作用中断引起的。Myc蛋白最重要的生物学干扰之一是其与Max蛋白(转录因子家族的另一重要因子)的二聚作用。这种异二聚体与E-Boxs的结合,即增强子盒,作为一些在真核生物中作为蛋白质结合位点并已发现调节基因表达的DNA响应元件,被中断以调节癌症的发病机理。强效的小分子Myc-Max二聚化抑制剂的全身有效性受到不良的生物利用度,快速的新陈代谢和不足的目标位点渗透的限制。通过成功合成智能货物,可以完全实现靶向Myc的基因疗法的潜力。我们从氨基甲酰基-生物共轭的乙酰辅酶-A的纳米组装中开发了一种“核素”型纳米颗粒“ siNozyme”(45±5 nm),用于稳定结合化学疗法和生物制剂以实现对人类黑素瘤的显着抑制。结果表明,以拓扑异构酶抑制剂,阿莫那肽的代码传递作用与siRNA靶向转录基因cMyc导致约90%的生长抑制和95%的蛋白质抑制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号