当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Ligand, Metal, and Solvation on the Structure and Stability of Contact Ion Pairs Relevant to Olefin Polymerization Catalyzed by Rare-Earth-Metal Complexes: A DFT Study
Organometallics ( IF 2.5 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acs.organomet.7b00857 Xingbao Wang 1, 2 , Guangli Zhou 1 , Bo Liu 3 , Yi Luo 1
Organometallics ( IF 2.5 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acs.organomet.7b00857 Xingbao Wang 1, 2 , Guangli Zhou 1 , Bo Liu 3 , Yi Luo 1
Affiliation
|
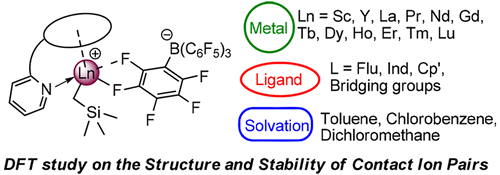
A full account of theoretical analyses at the DFT level has been reported, focusing on the formation and reactivity of a family of cationic [R-(CH2)n-Py-Sc(CH2SiMe3)]+ catalysts and the effects of counterion and solvation. Two sets of model systems have been considered: (a) structures having identical bridging unit (n = 1) but having varying cyclopentadienyl groups (R = Cp′ (1), R = Ind (2), and R = Flu (3)) and (b) systems with the identical cyclopentadienyl moiety (Flu) but with varying bridging groups (n = 1 (3), n = 0 (4), and n = 2 (5)). For complex 3, various metal ions (Sc, Y, La, Pr, Nd, Gd, Tb, Dy, Ho, Er, Tm, and Lu) were considered to investigate the effect of central metals on the contact ion pairs (CIP). The formation and separation of CIP were found to be influenced by the steric hindrance of the ligand, the electron-donating ability of the cyclopentadienyl group, and the rare-earth-metal ion radius. The separation enthalpy of the CIPs decreases with increasing dielectronic constant of the solvent. The solvation hardly affects the energy barrier for styrene insertion into the Sc–C17H19 bond of the CIP [(Flu-CH2-Py)Sc-(C17H19)][B(C6F5)4]. A bulkier and more electron donating ancillary ligand, a smaller ion radius of the rare-earth metal, and a greater polarity of the solvent are more beneficial to the separation of CIP and thus to the monomer coordination, which could contribute to the improvement of polymerization activity.
中文翻译:

稀土金属络合物催化的配体,金属和溶剂化物对与烯烃聚合相关的接触离子对结构和稳定性的影响:DFT研究
已经报道了DFT级理论分析的全部内容,重点是一类阳离子[R-(CH 2)n -Py-Sc(CH 2 SiMe 3)] +催化剂的形成和反应性以及抗衡和溶剂化。已经考虑了两组模型系统:(a)具有相同桥接单元(n = 1)但具有变化的环戊二烯基的结构(R = Cp'(1),R = Ind(2)和R = Flu(3) )和(b)具有相同环戊二烯基部分(Flu)但具有不同桥接基团的系统(n = 1(3),n = 0(4),n = 2(5))。对于络合物3,考虑了各种金属离子(Sc,Y,La,Pr,Nd,Gd,Tb,Dy,Ho,Er,Tm和Lu)来研究中心金属对接触离子对(CIP)的影响。发现CIP的形成和分离受配体的空间位阻,环戊二烯基的给电子能力和稀土金属离子半径的影响。CIP的分离焓随溶剂双电子常数的增加而降低。溶剂化几乎不影响的能量势垒为苯乙烯插入钪-C 17 ħ 19的CIP [(流感-CH的键2 -py)SC-(C 17 ħ 19)] [B(C 6 F 5)4 ]。更大且给电子的辅助配体更大,稀土金属的离子半径更小,溶剂的极性更大,更有利于CIP的分离,从而有助于单体的配位,从而有助于聚合的改善。活动。
更新日期:2018-02-22
中文翻译:

稀土金属络合物催化的配体,金属和溶剂化物对与烯烃聚合相关的接触离子对结构和稳定性的影响:DFT研究
已经报道了DFT级理论分析的全部内容,重点是一类阳离子[R-(CH 2)n -Py-Sc(CH 2 SiMe 3)] +催化剂的形成和反应性以及抗衡和溶剂化。已经考虑了两组模型系统:(a)具有相同桥接单元(n = 1)但具有变化的环戊二烯基的结构(R = Cp'(1),R = Ind(2)和R = Flu(3) )和(b)具有相同环戊二烯基部分(Flu)但具有不同桥接基团的系统(n = 1(3),n = 0(4),n = 2(5))。对于络合物3,考虑了各种金属离子(Sc,Y,La,Pr,Nd,Gd,Tb,Dy,Ho,Er,Tm和Lu)来研究中心金属对接触离子对(CIP)的影响。发现CIP的形成和分离受配体的空间位阻,环戊二烯基的给电子能力和稀土金属离子半径的影响。CIP的分离焓随溶剂双电子常数的增加而降低。溶剂化几乎不影响的能量势垒为苯乙烯插入钪-C 17 ħ 19的CIP [(流感-CH的键2 -py)SC-(C 17 ħ 19)] [B(C 6 F 5)4 ]。更大且给电子的辅助配体更大,稀土金属的离子半径更小,溶剂的极性更大,更有利于CIP的分离,从而有助于单体的配位,从而有助于聚合的改善。活动。











































 京公网安备 11010802027423号
京公网安备 11010802027423号