当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Control Over Silicone Synthesis using SiH Chemistry: The Piers–Rubinsztajn Reaction
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-03-30 , DOI: 10.1002/chem.201800123 Michael A. Brook 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-03-30 , DOI: 10.1002/chem.201800123 Michael A. Brook 1
Affiliation
|
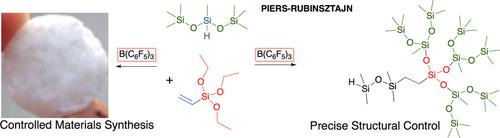
There is a strong imperative to synthesize polymers with highly controlled structures and narrow property ranges. Silicone polymers do not lend themselves to this paradigm because acids or bases lead to siloxane equilibration and loss of structure. By contrast, elegant levels of control are possible when using the Piers–Rubinsztajn reaction and analogues, in which the hydrophobic, strong Lewis acid B(C6F5)3 activates SiH groups, permitting the synthesis of precise siloxanes under mild conditions in high yield; siloxane decomposition processes are slow under these conditions. A broad range of oxygen nucleophiles including alkoxysilanes, silanols, phenols, and aryl alkyl ethers participate in the reaction to create elastomers, foams and green composites, for example, derived from lignin. In addition, the process permits the synthesis of monofunctional dendrons that can be assembled into larger entities including highly branched silicones and dendrimers either using the Piers–Rubinsztajn process alone, or in combination with hydrosilylation or other orthogonal reactions.
中文翻译:

SiH化学合成有机硅的新控制方法:Piers–Rubinsztajn反应
迫切需要合成具有高度受控结构和狭窄性能范围的聚合物。有机硅聚合物不适合这种范例,因为酸或碱会导致硅氧烷平衡并失去结构。相比之下,当使用Piers-Rubinsztajn反应及其类似物时,可能需要优雅的控制水平,其中疏水性强路易斯酸B(C 6 F 5)3活化SiH基团,允许在温和条件下以高收率合成精确的硅氧烷;在这些条件下,硅氧烷的分解过程很慢。各种各样的氧亲核试剂,包括烷氧基硅烷,硅烷醇,苯酚和芳基烷基醚,参与反应以生成弹性体,泡沫和绿色复合材料,例如,衍生自木质素。此外,该方法还可以合成单官能树枝状分子,这些树枝状分子可以单独使用Piers-Rubinsztajn方法,或者与氢化硅烷化或其他正交反应结合,组装成更大的实体,包括高度支化的有机硅和树枝状聚合物。
更新日期:2018-03-30
中文翻译:

SiH化学合成有机硅的新控制方法:Piers–Rubinsztajn反应
迫切需要合成具有高度受控结构和狭窄性能范围的聚合物。有机硅聚合物不适合这种范例,因为酸或碱会导致硅氧烷平衡并失去结构。相比之下,当使用Piers-Rubinsztajn反应及其类似物时,可能需要优雅的控制水平,其中疏水性强路易斯酸B(C 6 F 5)3活化SiH基团,允许在温和条件下以高收率合成精确的硅氧烷;在这些条件下,硅氧烷的分解过程很慢。各种各样的氧亲核试剂,包括烷氧基硅烷,硅烷醇,苯酚和芳基烷基醚,参与反应以生成弹性体,泡沫和绿色复合材料,例如,衍生自木质素。此外,该方法还可以合成单官能树枝状分子,这些树枝状分子可以单独使用Piers-Rubinsztajn方法,或者与氢化硅烷化或其他正交反应结合,组装成更大的实体,包括高度支化的有机硅和树枝状聚合物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号