Molecular Catalysis ( IF 3.9 ) Pub Date : 2018-02-21 , DOI: 10.1016/j.mcat.2018.01.022 Zhanao Lv , Cholho Choe , Yunfeng Wu , Haibin Wang , Zhuqi Chen , Guangxing Li , Guochuan Yin
|
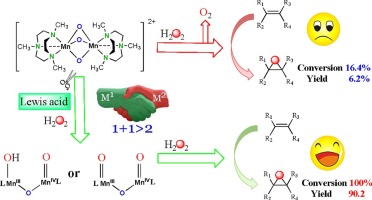
This work demonstrates a novel strategy that the introduction of non-redox metal ions as Lewis acids to the classic dinuclear manganese complex [MnIV2(μ-O)3(Me3tacn)2](PF6)2 can greatly promote the alkene epoxidation efficiency under mild conditions with H2O2 as the solely terminal oxidant because of its economic and environmental advantages. When [MnIV2(μ-O)3(Me3tacn)2](PF6)2 was used as the catalyst in the absence of Lewis acids, only 16.4% conversion of cyclooctene with 6.2% yield of epoxide was obtained and the obvious decomposition of H2O2 was observed. However, the oxygen transfer efficiency of the catalyst was sharply improved with 100% conversion and 90.2% yield of epoxide under identical conditions when the non-redox metal ion, such as Sc3+, was introduced to the catalytic system. The novel strategy was successfully applied to the epoxidation reactions of different types of alkenes. Through UV–vis, FT-IR, EPR and CV characterizations, it was evidenced that the non-redox metal ions with high positive charge as Lewis acids could dissociate the sluggish dinuclear Mn-(μ-O)3-Mn core and the open-loop dinuclear manganese complex, HO-MnIII-(μ-O)-MnIV = O or O = MnIV-(μ-O)-MnIV = O, was proposed as the active species, which was capable of the alkene epoxidation process. This work illustrated an alternative protocol to manipulate the reactivity of those sluggish catalysts by the introduction of non-redox metal ions and provided clues to understand the role of non-redox metal ions in metalloenzymes and heterogeneous catalysts.
中文翻译:

非氧化还原金属离子通过以H 2 O 2为氧源的Mn-Me 3 tacn配合物促进氧原子的转移
这项工作表明了一种新的策略,即将非氧化还原金属离子作为路易斯酸引入经典的双核锰配合物[Mn IV 2(μ-O)3(Me 3 tacn)2 ](PF 6)2可以极大地促进该过程。 H 2 O 2作为唯一末端氧化剂,在温和条件下的烯烃环氧化效率,因为其经济和环境优势。当[Mn IV 2(μ-O)3(Me 3 tacn)2 ](PF 6)2在没有路易斯酸的情况下,将其用作催化剂,仅获得16.4%的环辛烯转化率和6.2%的环氧化物收率,并且观察到H 2 O 2明显分解。但是,当将非氧化还原金属离子(例如Sc 3+)引入到催化体系中时,在相同的条件下,转化率达到100%,环氧化物的收率达到90.2%,催化剂的氧转移效率急剧提高。该新策略已成功应用于不同类型烯烃的环氧化反应。通过UV-vis,FT-IR,EPR和CV表征,证明具有高正电荷的非氧化还原金属离子(路易斯酸)可以分解缓慢的双核Mn-(μ-O)3-Mn核和开环双核锰配合物HO-Mn III-(μ-O)-Mn IV = O或O = Mn IV-(μ-O)-Mn IV = O被提议为活性物种,它能够进行烯烃环氧化过程。这项工作说明了通过引入非氧化还原金属离子来控制那些反应迟钝的催化剂的反应性的替代方案,并为了解非氧化还原金属离子在金属酶和非均相催化剂中的作用提供了线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号