Molecular Catalysis ( IF 3.9 ) Pub Date : 2018-02-21 , DOI: 10.1016/j.mcat.2018.01.030 Pranab Kumar Rakshit , Ravi Kumar Voolapalli , Sreedevi Upadhyayula
|
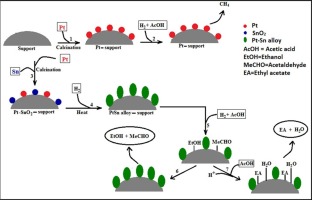
Gas phase hydrogenation of acetic acid was investigated over a series of SiO2-Al2O3 supported platinum-tin (Pt-Sn) catalysts. The active metals were impregnated over the support using incipient wetness technique and the resulting catalyst samples were characterized by Transmission electron microscopy, Hydrogen pulse chemisorption, BET surface area analyzer, Powder X-Ray diffraction, NH3-Temperature programmed desorption and H2-Temperature programmed reduction methods. Acetic acid hydrogenation reaction was carried out in an isothermal fixed bed catalyst testing unit. The results revealed that bimetallic Pt-Sn catalyst forms Pt-Sn alloy upon reduction which favors acetic acid hydrogenation to ethanol compared to competing side product CH4. The magnitude of Pt-Sn alloy formed per unit mass of catalyst depends upon the Pt/ Sn molar ratio in the calcined catalyst sample. 3 wt% Pt- 3 wt% Sn on SiO2-Al2O3 was found to be the optimum catalyst loading, resulting in 81% acetic acid conversion with 95% ethanol selectivity at 2 MPa and 270 °C. Further increase in ethanol selectivity would require prevention of esterification of acetic acid with ethanol, which leads to formation of ethyl acetate as by-product. The effect of catalyst acidity on acetic acid conversion and ethanol selectivity was studied and it was observed that proton donating capability of the support leads to the formation of ethyl acetate as by-product which, in turn, reduces ethanol selectivity. The ethanol synthesis reaction and esterification reaction over Bronsted acid sites takes place in series. The rate of esterification reaction was found to be highly dependent on the Bronsted acid density of the catalysts. Other catalyst parameters have little role on ethyl acetate yield.
中文翻译:

负载型Pt-Sn催化剂上乙酸加氢制乙醇:布朗斯台德酸度对产物选择性的影响
在一系列SiO 2 -Al 2 O 3负载的铂-锡(Pt-Sn)催化剂上研究了乙酸的气相加氢。使用初期润湿技术将活性金属浸渍在载体上,并通过透射电子显微镜,氢脉冲化学吸附,BET表面积分析仪,粉末X射线衍射,NH 3-程序升温脱附和H 2表征所得催化剂样品。-温度编程的还原方法。乙酸加氢反应在等温固定床催化剂测试装置中进行。结果表明,双金属Pt-Sn催化剂在还原时形成Pt-Sn合金,与竞争性副产物CH 4相比,有利于乙酸加氢成乙醇。每单位质量催化剂形成的Pt-Sn合金的大小取决于煅烧催化剂样品中的Pt / Sn摩尔比。SiO 2 -Al 2 O 3上3 wt%Pt- 3 wt%Sn据发现,这是最佳的催化剂负载量,在2 MPa和270°C下可产生81%的乙酸转化率和95%的乙醇选择性。乙醇选择性的进一步提高将需要防止乙酸与乙醇的酯化,这导致形成副产物乙酸乙酯。研究了催化剂酸度对乙酸转化率和乙醇选择性的影响,并且观察到载体的质子给体能力导致形成副产物乙酸乙酯,这反过来降低了乙醇选择性。布朗斯台德酸位上的乙醇合成反应和酯化反应是串联进行的。发现酯化反应的速率高度依赖于催化剂的布朗斯台德酸密度。其他催化剂参数对乙酸乙酯收率影响很小。











































 京公网安备 11010802027423号
京公网安备 11010802027423号