Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-02-21 , DOI: 10.1016/j.cplett.2018.02.038 Chang Q. Sun , Jiasheng Chen , Chuang Yao , Xinjuan Liu , Xi Zhang , Yongli Huang
|
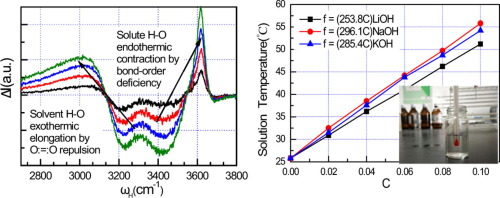
The resultant energy of solvent H-O bond exothermic elongation by O:⇔:O repulsion, featured at <3100 cm-1, and the solute H-O bond endothermic contraction by bond-order-deficiency, at 3610 cm-1, heats up the (Li, Na, K)OH solutions. The solution temperature increases linearly with the number fraction of the ordinary O:H-O bonds transiting into their hydration states. The elongated H-O bond emits >150% the O:H cohesive energy of 0.095 eV that caps the energy dissipating by molecular motion, thermal fluctuation, diffusion, and even evaporation. Therefore, the intramolecular H-O bond relaxation dictates the OH- solvation bonding thermodynamics and the performance of basic solutions.
中文翻译:

(Li,Na,K)OH水化热力学:溶液自加热
溶剂HO键因O:⇔:O排斥而放热伸长的合成能量在<3100 cm -1处发生,溶质HO键通过键序缺陷在3610 cm -1处发生吸热收缩,使(Li ,Na,K)OH溶液。溶液温度随着普通O:HO键的数量分数转变为水合状态而线性增加。细长的HO键发出的O:H内聚能为0.095 eV,大于150%,该内能限制了通过分子运动,热涨落,扩散甚至蒸发而耗散的能量。因此,分子内HO键松弛使然的OH -溶剂化结合热力学和碱性溶液的性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号