当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Excitation-Wavelength-Dependent Photocycle Initiation Dynamics Resolve Heterogeneity in the Photoactive Yellow Protein from Halorhodospira halophila
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acs.biochem.7b01114 L. Tyler Mix 1 , Elizabeth C. Carroll 1 , Dmitry Morozov 2 , Jie Pan 1 , Wendy Ryan Gordon , Andrew Philip , Jack Fuzell 1 , Masato Kumauchi 3 , Ivo van Stokkum 4 , Gerrit Groenhof 2 , Wouter D. Hoff 3 , Delmar S. Larsen 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acs.biochem.7b01114 L. Tyler Mix 1 , Elizabeth C. Carroll 1 , Dmitry Morozov 2 , Jie Pan 1 , Wendy Ryan Gordon , Andrew Philip , Jack Fuzell 1 , Masato Kumauchi 3 , Ivo van Stokkum 4 , Gerrit Groenhof 2 , Wouter D. Hoff 3 , Delmar S. Larsen 1
Affiliation
|
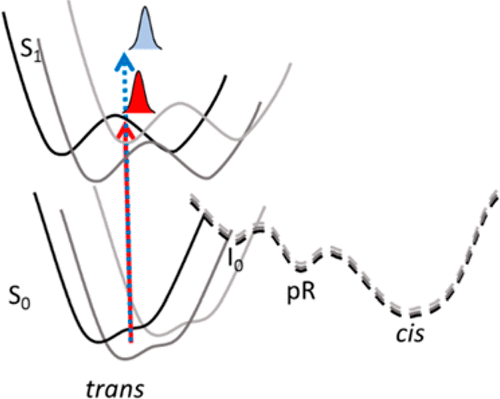
Photoactive yellow proteins (PYPs) make up a diverse class of blue-light-absorbing bacterial photoreceptors. Electronic excitation of the p-coumaric acid chromophore covalently bound within PYP results in triphasic quenching kinetics; however, the molecular basis of this behavior remains unresolved. Here we explore this question by examining the excitation-wavelength dependence of the photodynamics of the PYP from Halorhodospira halophila via a combined experimental and computational approach. The fluorescence quantum yield, steady-state fluorescence emission maximum, and cryotrapping spectra are demonstrated to depend on excitation wavelength. We also compare the femtosecond photodynamics in PYP at two excitation wavelengths (435 and 475 nm) with a dual-excitation-wavelength-interleaved pump–probe technique. Multicompartment global analysis of these data demonstrates that the excited-state photochemistry of PYP depends subtly, but convincingly, on excitation wavelength with similar kinetics with distinctly different spectral features, including a shifted ground-state beach and altered stimulated emission oscillator strengths and peak positions. Three models involving multiple excited states, vibrationally enhanced barrier crossing, and inhomogeneity are proposed to interpret the observed excitation-wavelength dependence of the data. Conformational heterogeneity was identified as the most probable model, which was supported with molecular mechanics simulations that identified two levels of inhomogeneity involving the orientation of the R52 residue and different hydrogen bonding networks with the p-coumaric acid chromophore. Quantum calculations were used to confirm that these inhomogeneities track to altered spectral properties consistent with the experimental results.
中文翻译:

激发波长依赖的光周期起始动力学解决了嗜盐卤单螺旋藻光敏黄色蛋白中的异质性。
光敏黄色蛋白(PYP)构成了吸收蓝光的细菌感光体的不同类别。PYP内共价结合的对香豆酸生色团的电子激发导致三相猝灭动力学。但是,这种行为的分子基础仍未解决。在这里,我们通过检查嗜盐嗜盐菌的PYP的光动力的激发波长依赖性来探讨这个问题。通过实验和计算相结合的方法。荧光量子产率,稳态荧光发射最大值和冷冻谱表明,取决于激发波长。我们还使用双激发波长交错泵浦探针技术比较了在两个激发波长(435和475 nm)下PYP中的飞秒光动力学。这些数据的多室整体分析表明,PYP的激发态光化学微妙地但有说服力地取决于具有相似动力学的激发波长,具有明显不同的光谱特征,包括偏移的基态海滩和变化的受激发射振荡器强度和峰值位置。三种模型涉及多个激发态,振动增强的势垒穿越,提出了非均质性和非均质性来解释观测到的数据的激发波长依赖性。构象异质性被认为是最可能的模型,这得到了分子力学模拟的支持,该分子力学模拟确定了两个水平的不均匀性,包括R52残基的取向以及与氢原子相关的不同氢键网络。对香豆酸发色团。量子计算被用来确认这些不均匀性追踪到改变后的光谱特性,与实验结果一致。
更新日期:2018-02-21
中文翻译:

激发波长依赖的光周期起始动力学解决了嗜盐卤单螺旋藻光敏黄色蛋白中的异质性。
光敏黄色蛋白(PYP)构成了吸收蓝光的细菌感光体的不同类别。PYP内共价结合的对香豆酸生色团的电子激发导致三相猝灭动力学。但是,这种行为的分子基础仍未解决。在这里,我们通过检查嗜盐嗜盐菌的PYP的光动力的激发波长依赖性来探讨这个问题。通过实验和计算相结合的方法。荧光量子产率,稳态荧光发射最大值和冷冻谱表明,取决于激发波长。我们还使用双激发波长交错泵浦探针技术比较了在两个激发波长(435和475 nm)下PYP中的飞秒光动力学。这些数据的多室整体分析表明,PYP的激发态光化学微妙地但有说服力地取决于具有相似动力学的激发波长,具有明显不同的光谱特征,包括偏移的基态海滩和变化的受激发射振荡器强度和峰值位置。三种模型涉及多个激发态,振动增强的势垒穿越,提出了非均质性和非均质性来解释观测到的数据的激发波长依赖性。构象异质性被认为是最可能的模型,这得到了分子力学模拟的支持,该分子力学模拟确定了两个水平的不均匀性,包括R52残基的取向以及与氢原子相关的不同氢键网络。对香豆酸发色团。量子计算被用来确认这些不均匀性追踪到改变后的光谱特性,与实验结果一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号