当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Coordinate, Free Energy, and Rate of Intramolecular Proton Transfer in Human Carbonic Anhydrase II
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acs.jpcb.7b10713 Sanjib Paul 1 , Tanmoy Kumar Paul 1 , Srabani Taraphder 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1021/acs.jpcb.7b10713 Sanjib Paul 1 , Tanmoy Kumar Paul 1 , Srabani Taraphder 1
Affiliation
|
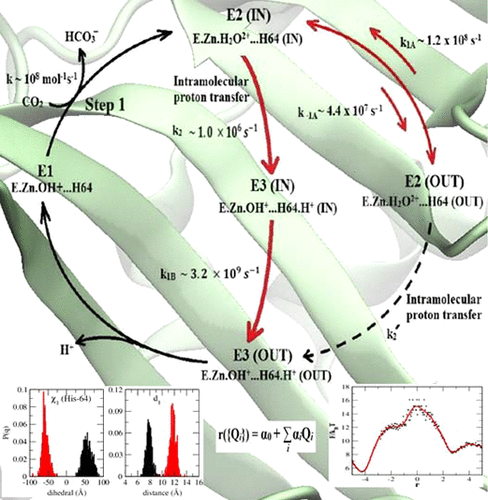
The role of structure and dynamics of an enzyme has been investigated at three different stages of its function including the chemical event it catalyzes. A one-pot computational method has been designed for each of these stages on the basis of classical and/or quantum mechanical–molecular mechanical molecular dynamics and transition path sampling simulations. For a pair of initial and final states A and B separated by a high free-energy barrier, using a two-stage selection process, several collective variables (CVs) are identified that can delineate A and B. However, these CVs are found to exhibit strong cross-coupling over the transition paths. A set of mutually orthogonal order parameters is then derived from these CVs and an optimal reaction coordinate, r, determined applying half-trajectory likelihood maximization along with a Bayesian information criterion. The transition paths are also used to project the multidimensional free energy surface and barrier crossing dynamics along r. The proposed scheme has been applied to the rate-determining intramolecular proton transfer reaction of the well-known enzyme human carbonic anhydrase II. The potential of mean force, F(r), in the absence of the chemical step is found to reproduce earlier results on the equilibrium population of two side-chain orientations of key residue His-64. Estimation of rate constants, k, from mean first passage times for the three different stages of catalysis shows that the rate-determining step of intramolecular proton transfer occurs with k ≃ 1.0 × 106 s–1, in close agreement with known experimental results.
中文翻译:

人类碳酸酐酶II中的反应坐标,自由能和分子内质子转移速率
已经在酶功能的三个不同阶段(包括其催化的化学事件)研究了酶的结构和动力学的作用。在经典和/或量子力学-分子机械分子动力学和过渡路径采样模拟的基础上,针对这些阶段的每一个设计了一种一锅法计算方法。对于通过高自由能垒隔开的一对初始状态A和最终状态B,使用两阶段选择过程,可以识别出几个可以描述A和B的集体变量(CV)。但是,发现这些CV可以在过渡路径上表现出很强的交叉耦合。然后,从这些CV和最佳反应坐标r得出一组相互正交的顺序参数确定,应用半轨迹似然最大化以及贝叶斯信息准则。过渡路径还用于沿r投影多维自由能表面和势垒穿越动力学。所提出的方案已应用于确定酶人碳酸酐酶II的分子内质子转移反应的速率。发现在没有化学步骤的情况下,平均力F(r)的潜力可在关键残基His-64的两个侧链方向的平衡种群上重现较早的结果。速率常数的估计,k从平均第一通道时间的催化示出了三个不同的阶段即分子内质子转移的速率决定步骤与发生ķ ≃1.0×10 6小号-1,与已知的实验结果非常一致。
更新日期:2018-02-21
中文翻译:

人类碳酸酐酶II中的反应坐标,自由能和分子内质子转移速率
已经在酶功能的三个不同阶段(包括其催化的化学事件)研究了酶的结构和动力学的作用。在经典和/或量子力学-分子机械分子动力学和过渡路径采样模拟的基础上,针对这些阶段的每一个设计了一种一锅法计算方法。对于通过高自由能垒隔开的一对初始状态A和最终状态B,使用两阶段选择过程,可以识别出几个可以描述A和B的集体变量(CV)。但是,发现这些CV可以在过渡路径上表现出很强的交叉耦合。然后,从这些CV和最佳反应坐标r得出一组相互正交的顺序参数确定,应用半轨迹似然最大化以及贝叶斯信息准则。过渡路径还用于沿r投影多维自由能表面和势垒穿越动力学。所提出的方案已应用于确定酶人碳酸酐酶II的分子内质子转移反应的速率。发现在没有化学步骤的情况下,平均力F(r)的潜力可在关键残基His-64的两个侧链方向的平衡种群上重现较早的结果。速率常数的估计,k从平均第一通道时间的催化示出了三个不同的阶段即分子内质子转移的速率决定步骤与发生ķ ≃1.0×10 6小号-1,与已知的实验结果非常一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号