当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid access to diverse, trifluoromethyl-substituted alkenes using complementary strategies†
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-22 00:00:00 , DOI: 10.1039/c7sc05420c James P Phelan 1 , Rebecca J Wiles 1 , Simon B Lang 1 , Christopher B Kelly 1 , Gary A Molander 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-22 00:00:00 , DOI: 10.1039/c7sc05420c James P Phelan 1 , Rebecca J Wiles 1 , Simon B Lang 1 , Christopher B Kelly 1 , Gary A Molander 1
Affiliation
|
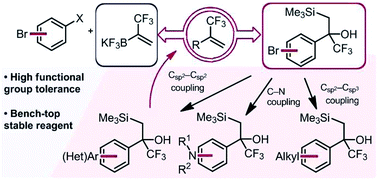
Two synergistic approaches to the facile assembly of complex α-trifluoromethyl alkenes are described. Using α-trifluoromethyl-β-silyl alcohols as masked trifluoromethyl alkenes, cross-coupling or related functionalization processes at distal electrophilic sites can be executed without inducing Peterson elimination. Subsequent Lewis acidic activation affords functionalized α-trifluoromethyl alkenes. Likewise, the development of a novel α-trifluoromethylvinyl trifluoroborate reagent complements this approach and allows a one-step cross-coupling of (hetero)aryl halides to access a broad array of complex α-trifluoromethyl alkenes.
中文翻译:

使用互补策略快速获取多种三氟甲基取代的烯烃†
描述了两种轻松组装复杂 α-三氟甲基烯烃的协同方法。使用α-三氟甲基-β-甲硅烷基醇作为掩蔽三氟甲基烯烃,可以在远端亲电子位点进行交叉偶联或相关功能化过程,而不会引起Peterson消除。随后的路易斯酸活化得到官能化的α-三氟甲基烯烃。同样,一种新型 α-三氟甲基乙烯基三氟硼酸酯试剂的开发补充了这一方法,并允许(杂)芳基卤化物的一步交叉偶联以获得多种复杂的 α-三氟甲基烯烃。
更新日期:2018-02-22
中文翻译:

使用互补策略快速获取多种三氟甲基取代的烯烃†
描述了两种轻松组装复杂 α-三氟甲基烯烃的协同方法。使用α-三氟甲基-β-甲硅烷基醇作为掩蔽三氟甲基烯烃,可以在远端亲电子位点进行交叉偶联或相关功能化过程,而不会引起Peterson消除。随后的路易斯酸活化得到官能化的α-三氟甲基烯烃。同样,一种新型 α-三氟甲基乙烯基三氟硼酸酯试剂的开发补充了这一方法,并允许(杂)芳基卤化物的一步交叉偶联以获得多种复杂的 α-三氟甲基烯烃。










































 京公网安备 11010802027423号
京公网安备 11010802027423号