当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Positional effects of second-sphere amide pendants on electrochemical CO2 reduction catalyzed by iron porphyrins†
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1039/c7sc04682k Eva M Nichols 1, 2 , Jeffrey S Derrick 1, 2 , Sepand K Nistanaki 1 , Peter T Smith 1, 2 , Christopher J Chang 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-21 00:00:00 , DOI: 10.1039/c7sc04682k Eva M Nichols 1, 2 , Jeffrey S Derrick 1, 2 , Sepand K Nistanaki 1 , Peter T Smith 1, 2 , Christopher J Chang 1, 2, 3, 4
Affiliation
|
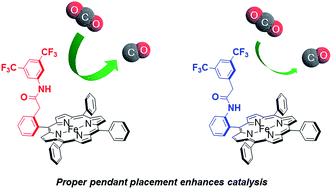
The development of catalysts for electrochemical reduction of carbon dioxide offers an attractive approach to transforming this greenhouse gas into value-added carbon products with sustainable energy input. Inspired by natural bioinorganic systems that feature precisely positioned hydrogen-bond donors in the secondary coordination sphere to direct chemical transformations occurring at redox-active metal centers, we now report the design, synthesis, and characterization of a series of iron tetraphenylporphyrin (Fe-TPP) derivatives bearing amide pendants at various positions at the periphery of the metal core. Proper positioning of the amide pendants greatly affects the electrocatalytic activity for carbon dioxide reduction to carbon monoxide. In particular, derivatives bearing proximal and distal amide pendants on the ortho position of the phenyl ring exhibit significantly larger turnover frequencies (TOF) compared to the analogous para-functionalized amide isomers or unfunctionalized Fe-TPP. Analysis of TOF as a function of catalyst standard reduction potential enables first-sphere electronic effects to be disentangled from second-sphere through-space interactions, suggesting that the ortho-functionalized porphyrins can utilize the latter second-sphere property to promote CO2 reduction. Indeed, the distally-functionalized ortho-amide isomer shows a significantly larger through-space interaction than its proximal ortho-amide analogue. These data establish that proper positioning of secondary coordination sphere groups is an effective design element for breaking electronic scaling relationships that are often observed in electrochemical CO2 reduction.
中文翻译:

第二球酰胺悬挂物对铁卟啉催化的电化学 CO2 还原的位置效应†
用于二氧化碳电化学还原的催化剂的开发提供了一种有吸引力的方法,可以将这种温室气体转化为具有可持续能源输入的增值碳产品。受天然生物无机系统的启发,该系统在二次配位层中精确定位氢键供体,以指导氧化还原活性金属中心发生的化学转化,我们现在报告了一系列四苯基铁卟啉(Fe-TPP)的设计、合成和表征。 ) 衍生物在金属核外围的不同位置带有酰胺垂饰。酰胺侧链的正确定位极大地影响二氧化碳还原为一氧化碳的电催化活性。特别地,与类似的对位官能化酰胺异构体或未官能化Fe-TPP相比,在苯环的邻位上带有近端和远端酰胺侧基的衍生物表现出显着更大的转换频率(TOF )。TOF 分析作为催化剂标准还原电位的函数,使得第一球体的电子效应能够与第二球体的空间相互作用分离,这表明邻位官能化的卟啉可以利用后者的第二球体特性来促进 CO 2还原。事实上,远端官能化的邻酰胺异构体比其近端邻酰胺类似物表现出明显更大的空间相互作用。这些数据表明,二次配位球基团的正确定位是打破电化学CO 2还原中经常观察到的电子缩放关系的有效设计元素。
更新日期:2018-02-21
中文翻译:

第二球酰胺悬挂物对铁卟啉催化的电化学 CO2 还原的位置效应†
用于二氧化碳电化学还原的催化剂的开发提供了一种有吸引力的方法,可以将这种温室气体转化为具有可持续能源输入的增值碳产品。受天然生物无机系统的启发,该系统在二次配位层中精确定位氢键供体,以指导氧化还原活性金属中心发生的化学转化,我们现在报告了一系列四苯基铁卟啉(Fe-TPP)的设计、合成和表征。 ) 衍生物在金属核外围的不同位置带有酰胺垂饰。酰胺侧链的正确定位极大地影响二氧化碳还原为一氧化碳的电催化活性。特别地,与类似的对位官能化酰胺异构体或未官能化Fe-TPP相比,在苯环的邻位上带有近端和远端酰胺侧基的衍生物表现出显着更大的转换频率(TOF )。TOF 分析作为催化剂标准还原电位的函数,使得第一球体的电子效应能够与第二球体的空间相互作用分离,这表明邻位官能化的卟啉可以利用后者的第二球体特性来促进 CO 2还原。事实上,远端官能化的邻酰胺异构体比其近端邻酰胺类似物表现出明显更大的空间相互作用。这些数据表明,二次配位球基团的正确定位是打破电化学CO 2还原中经常观察到的电子缩放关系的有效设计元素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号