当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bio-based polymer networks by thiol-ene photopolymerization of allylated L-glutamic acids and L-tyrosines
European Polymer Journal ( IF 5.8 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.eurpolymj.2018.02.027 Shima Aoyagi , Toshiaki Shimasaki , Naozumi Teramoto , Mitsuhiro Shibata
European Polymer Journal ( IF 5.8 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.eurpolymj.2018.02.027 Shima Aoyagi , Toshiaki Shimasaki , Naozumi Teramoto , Mitsuhiro Shibata
|
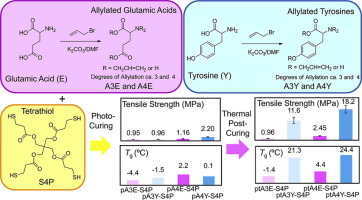
Abstract Triallylated l -glutamic acid and l -tyrosine (A3E amd A3Y) and their tetraallyl derivatives (A4E and A4Y) were synthesized by reactions of l -glutamic acid and l -tyrosine with allyl bromide in the presence of potassium carbonate, respectively. Thiol-ene photopolymerizations of the allylated amino acids and a pentaerythritol-based tetrathiol (S4P) at allyl/SH 1/1 in the presence of photo-initiators produced photo-cured products (pA3E-S4P, pA3Y-S4P, pA4E-S4P and pA4Y-S4P). Furthermore, thermal post-curing after photo-curing of allylated amino acid/S4P mixtures containing photo-initiators and a thermal initiator produced photo and thermal cured products (ptA3E-S4P, ptA3Y-S4P, ptA4E-S4P and ptA4Y-S4P). Although the thermally post-cured products became dark in color, all the cured films were flat and had smooth surfaces. The FT-IR analysis revealed that allyl and thiol conversions increased by the thermal post-curing for all the curing systems. The glass transition temperature (Tg), 5% weight loss temperature (Td5), tensile strength and tensile modulus of each thermally post-cured product were higher than those of the corresponding photo-cured product, reflecting the increase of crosslinking density by the thermal post-curing. Also, A4E- and A4Y-based cured products exhibited higher Tgs, Td5s, tensile strengths and moduli than the corresponding A3E- and A3Y-based cured products, respectively. Especially, ptA4Y-S4P exhibited the highest Tg (24.4 °C), Td5 (319 °C), tensile strength (18.2 MPa) and tensile modulus (1037 MPa) among all the cured products.
中文翻译:

烯丙基化L-谷氨酸和L-酪氨酸硫醇-烯光聚合的生物基聚合物网络
摘要 在碳酸钾的存在下,分别通过L-谷氨酸和L-酪氨酸与烯丙基溴反应合成了三烯丙基化的L-谷氨酸和L-酪氨酸(A3E和A3Y)及其四烯丙基衍生物(A4E和A4Y)。在光引发剂存在下,烯丙基化氨基酸和季戊四醇基四硫醇 (S4P) 在烯丙基/SH 1/1 的硫醇-烯光聚合产生光固化产物(pA3E-S4P、pA3Y-S4P、pA4E-S4P 和pA4Y-S4P)。此外,含有光引发剂和热引发剂的烯丙基化氨基酸/S4P混合物在光固化后进行热后固化产生光固化产物和热固化产物(ptA3E-S4P、ptA3Y-S4P、ptA4E-S4P和ptA4Y-S4P)。尽管热后固化产品颜色变深,但所有固化膜都是平坦的并且具有光滑的表面。FT-IR 分析表明,所有固化体系的热后固化都增加了烯丙基和硫醇的转化率。每种热后固化产品的玻璃化转变温度(Tg)、5%失重温度(Td5)、拉伸强度和拉伸模量均高于相应的光固化产品,反映了热固化后交联密度的增加。后固化。此外,A4E 和 A4Y 基固化产品分别显示出比相应的 A3E 和 A3Y 基固化产品更高的 Tgs、Td5s、拉伸强度和模量。特别是,ptA4Y-S4P 在所有固化产品中表现出最高的 Tg (24.4 °C)、Td5 (319 °C)、拉伸强度 (18.2 MPa) 和拉伸模量 (1037 MPa)。每种热后固化产品的玻璃化转变温度(Tg)、5%失重温度(Td5)、拉伸强度和拉伸模量均高于相应的光固化产品,反映了热固化后交联密度的增加。后固化。此外,A4E 和 A4Y 基固化产品分别显示出比相应的 A3E 和 A3Y 基固化产品更高的 Tgs、Td5s、拉伸强度和模量。特别是,ptA4Y-S4P 在所有固化产品中表现出最高的 Tg (24.4 °C)、Td5 (319 °C)、拉伸强度 (18.2 MPa) 和拉伸模量 (1037 MPa)。每种热后固化产品的玻璃化转变温度(Tg)、5%失重温度(Td5)、拉伸强度和拉伸模量均高于相应的光固化产品,反映了热固化后交联密度的增加。后固化。此外,A4E 和 A4Y 基固化产品分别显示出比相应的 A3E 和 A3Y 基固化产品更高的 Tgs、Td5s、拉伸强度和模量。特别是,ptA4Y-S4P 在所有固化产品中表现出最高的 Tg (24.4 °C)、Td5 (319 °C)、拉伸强度 (18.2 MPa) 和拉伸模量 (1037 MPa)。反映了热后固化交联密度的增加。此外,A4E 和 A4Y 基固化产品分别显示出比相应的 A3E 和 A3Y 基固化产品更高的 Tgs、Td5s、拉伸强度和模量。特别是,ptA4Y-S4P 在所有固化产品中表现出最高的 Tg (24.4 °C)、Td5 (319 °C)、拉伸强度 (18.2 MPa) 和拉伸模量 (1037 MPa)。反映了热后固化交联密度的增加。此外,A4E 和 A4Y 基固化产品分别显示出比相应的 A3E 和 A3Y 基固化产品更高的 Tgs、Td5s、拉伸强度和模量。特别是,ptA4Y-S4P 在所有固化产品中表现出最高的 Tg (24.4 °C)、Td5 (319 °C)、拉伸强度 (18.2 MPa) 和拉伸模量 (1037 MPa)。
更新日期:2018-04-01
中文翻译:

烯丙基化L-谷氨酸和L-酪氨酸硫醇-烯光聚合的生物基聚合物网络
摘要 在碳酸钾的存在下,分别通过L-谷氨酸和L-酪氨酸与烯丙基溴反应合成了三烯丙基化的L-谷氨酸和L-酪氨酸(A3E和A3Y)及其四烯丙基衍生物(A4E和A4Y)。在光引发剂存在下,烯丙基化氨基酸和季戊四醇基四硫醇 (S4P) 在烯丙基/SH 1/1 的硫醇-烯光聚合产生光固化产物(pA3E-S4P、pA3Y-S4P、pA4E-S4P 和pA4Y-S4P)。此外,含有光引发剂和热引发剂的烯丙基化氨基酸/S4P混合物在光固化后进行热后固化产生光固化产物和热固化产物(ptA3E-S4P、ptA3Y-S4P、ptA4E-S4P和ptA4Y-S4P)。尽管热后固化产品颜色变深,但所有固化膜都是平坦的并且具有光滑的表面。FT-IR 分析表明,所有固化体系的热后固化都增加了烯丙基和硫醇的转化率。每种热后固化产品的玻璃化转变温度(Tg)、5%失重温度(Td5)、拉伸强度和拉伸模量均高于相应的光固化产品,反映了热固化后交联密度的增加。后固化。此外,A4E 和 A4Y 基固化产品分别显示出比相应的 A3E 和 A3Y 基固化产品更高的 Tgs、Td5s、拉伸强度和模量。特别是,ptA4Y-S4P 在所有固化产品中表现出最高的 Tg (24.4 °C)、Td5 (319 °C)、拉伸强度 (18.2 MPa) 和拉伸模量 (1037 MPa)。每种热后固化产品的玻璃化转变温度(Tg)、5%失重温度(Td5)、拉伸强度和拉伸模量均高于相应的光固化产品,反映了热固化后交联密度的增加。后固化。此外,A4E 和 A4Y 基固化产品分别显示出比相应的 A3E 和 A3Y 基固化产品更高的 Tgs、Td5s、拉伸强度和模量。特别是,ptA4Y-S4P 在所有固化产品中表现出最高的 Tg (24.4 °C)、Td5 (319 °C)、拉伸强度 (18.2 MPa) 和拉伸模量 (1037 MPa)。每种热后固化产品的玻璃化转变温度(Tg)、5%失重温度(Td5)、拉伸强度和拉伸模量均高于相应的光固化产品,反映了热固化后交联密度的增加。后固化。此外,A4E 和 A4Y 基固化产品分别显示出比相应的 A3E 和 A3Y 基固化产品更高的 Tgs、Td5s、拉伸强度和模量。特别是,ptA4Y-S4P 在所有固化产品中表现出最高的 Tg (24.4 °C)、Td5 (319 °C)、拉伸强度 (18.2 MPa) 和拉伸模量 (1037 MPa)。反映了热后固化交联密度的增加。此外,A4E 和 A4Y 基固化产品分别显示出比相应的 A3E 和 A3Y 基固化产品更高的 Tgs、Td5s、拉伸强度和模量。特别是,ptA4Y-S4P 在所有固化产品中表现出最高的 Tg (24.4 °C)、Td5 (319 °C)、拉伸强度 (18.2 MPa) 和拉伸模量 (1037 MPa)。反映了热后固化交联密度的增加。此外,A4E 和 A4Y 基固化产品分别显示出比相应的 A3E 和 A3Y 基固化产品更高的 Tgs、Td5s、拉伸强度和模量。特别是,ptA4Y-S4P 在所有固化产品中表现出最高的 Tg (24.4 °C)、Td5 (319 °C)、拉伸强度 (18.2 MPa) 和拉伸模量 (1037 MPa)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号