Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-02-22 , DOI: 10.1016/j.bmc.2018.02.035 Til Bahadur Thapa Magar , Seung Hee Seo , Tara Man Kadayat , Hyunji Jo , Aarajana Shrestha , Ganesh Bist , Pramila Katila , Youngjoo Kwon , Eung-Seok Lee
|
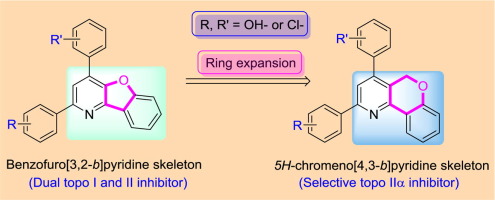
As part of our effort to develop potential topoisomerase IIα (topo IIα) targeting anticancer agents, we systematically designed a new series of hydroxy and chloro-substituted 2,4-diphenyl 5H-chromeno[4,3-b]pyridines. Total eighteen compounds were synthesized and tested for their ability to inhibit the function of topo I and IIα, and proliferation of human breast (T47D), colorectal (HCT15), and cervix (HeLa) cancer cells. Except compound 11, all of the tested compounds displayed selective topo IIα inhibitory activity. Compounds 8–18, 22, 24, and 25 showed excellent topo IIα inhibitory activity than a positive control, etoposide. Most of the compounds appeared to be superior to reference compounds in their antiproliferative activity. Structure-activity relationship (SAR) study has shown that it is better to place the hydroxyphenyl group at the 4-position of the central pyridine for superior topo IIα inhibition and antiproliferative activity. Similarly, the 3′-, or 4′-hydroxyphenyl substitution at the 2- and 4-positon of pyridine ring is important for better activity than 2′-substitution.
中文翻译:

新型羟基和氯取代的2,4-二苯基5 H -chromeno [4,3- b ]吡啶作为选择性拓扑异构酶IIα靶向抗癌药的合成与合成孔径雷达研究
作为我们开发潜在的靶向抗癌剂的拓扑异构酶IIα(拓扑IIα)的工作的一部分,我们系统地设计了一系列新的羟基和氯取代的2,4-二苯基5 H -chromeno [4,3- b ]吡啶。合成了总共18种化合物,并测试了它们抑制topo I和IIα功能以及抑制人乳腺癌(T47D),结直肠癌(HCT15)和子宫颈癌(HeLa)癌细胞增殖的能力。除化合物11外,所有测试的化合物均显示出选择性的topoIIα抑制活性。化合物8 - 18,22,24,和25与依托泊苷阳性对照相比,显示出优异的topoIIα抑制活性。大多数化合物的抗增殖活性似乎优于参考化合物。结构-活性关系(SAR)研究表明,最好在中央吡啶的4位上放置羟苯基,以获得更好的topoIIα抑制和抗增殖活性。类似地,在吡啶环的2-和4-位上的3'-或4'-羟基苯基取代对于比2'-取代更好的活性很重要。









































 京公网安备 11010802027423号
京公网安备 11010802027423号