当前位置:
X-MOL 学术
›
Anal. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a general method for quantifying IgG-based therapeutic monoclonal antibodies in human plasma using protein G purification coupled with a two internal standard calibration strategy using LC-MS/MS
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.aca.2018.02.040 Huai-Hsuan Chiu , Hsiao-Wei Liao , Yu-Yun Shao , Yen-Shen Lu , Ching-Hung Lin , I-Lin Tsai , Ching-Hua Kuo
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.aca.2018.02.040 Huai-Hsuan Chiu , Hsiao-Wei Liao , Yu-Yun Shao , Yen-Shen Lu , Ching-Hung Lin , I-Lin Tsai , Ching-Hua Kuo
|
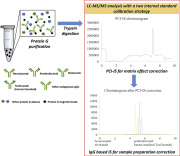
Monoclonal antibody (mAb) drugs have generated much interest in recent years for treating various diseases. Immunoglobulin G (IgG) represents a high percentage of mAb drugs that have been approved by the Food and Drug Administration (FDA). To facilitate therapeutic drug monitoring and pharmacokinetic/pharmacodynamic studies, we developed a general liquid chromatography-tandem mass spectrometry (LC-MS/MS) method to quantify the concentration of IgG-based mAbs in human plasma. Three IgG-based drugs (bevacizumab, nivolumab and pembrolizumab) were selected to demonstrate our method. Protein G beads were used for sample pretreatment due to their universal ability to trap IgG-based drugs. Surrogate peptides that were obtained after trypsin digestion were quantified by using LC-MS/MS. To calibrate sample preparation errors and matrix effects that occur during LC-MS/MS analysis, we used two internal standards (IS) method that include the IgG-based drug-IS tocilizumab and post-column infused IS. Using two internal standards was found to effectively improve quantification accuracy, which was within 15% for all mAb drugs that were tested at three different concentrations. This general method was validated in term of its precision, accuracy, linearity and sensitivity for 3 demonstration mAb drugs. The successful application of the method to clinical samples demonstrated its' applicability in clinical analysis. It is anticipated that this general method could be applied to other mAb-based drugs for use in precision medicine and clinical studies.
中文翻译:

使用蛋白质 G 纯化结合使用 LC-MS/MS 的两个内标校准策略,开发用于量化人血浆中基于 IgG 的治疗性单克隆抗体的通用方法
近年来,单克隆抗体 (mAb) 药物在治疗各种疾病方面引起了广泛关注。免疫球蛋白 G (IgG) 在获得食品和药物管理局 (FDA) 批准的 mAb 药物中占很大比例。为了促进治疗药物监测和药代动力学/药效学研究,我们开发了一种通用的液相色谱-串联质谱 (LC-MS/MS) 方法来量化人血浆中基于 IgG 的 mAb 的浓度。选择了三种基于 IgG 的药物(贝伐单抗、纳武单抗和派姆单抗)来证明我们的方法。由于 G 蛋白珠具有捕获基于 IgG 的药物的普遍能力,因此它们被用于样品预处理。通过使用 LC-MS/MS 对胰蛋白酶消化后获得的替代肽进行定量。为了校准 LC-MS/MS 分析过程中发生的样品制备误差和基质效应,我们使用了两种内标 (IS) 方法,包括基于 IgG 的药物 IS 托珠单抗和柱后灌注 IS。发现使用两种内标可有效提高定量准确度,在三种不同浓度下测试的所有 mAb 药物的准确度均在 15% 以内。该通用方法在精密度、准确度、线性和灵敏度方面对 3 种示范 mAb 药物进行了验证。该方法在临床样本中的成功应用证明了其在临床分析中的适用性。预计这种通用方法可以应用于其他基于 mAb 的药物,用于精准医学和临床研究。我们使用了两种内标 (IS) 方法,包括基于 IgG 的药物 IS 托珠单抗和柱后灌注 IS。发现使用两种内标可有效提高定量准确度,在三种不同浓度下测试的所有 mAb 药物的准确度均在 15% 以内。该通用方法在精密度、准确度、线性和灵敏度方面对 3 种示范 mAb 药物进行了验证。该方法在临床样本中的成功应用证明了其在临床分析中的适用性。预计这种通用方法可以应用于其他基于 mAb 的药物,用于精准医学和临床研究。我们使用了两种内标 (IS) 方法,包括基于 IgG 的药物 IS 托珠单抗和柱后灌注 IS。发现使用两种内标可有效提高定量准确度,在三种不同浓度下测试的所有 mAb 药物的准确度均在 15% 以内。该通用方法在精密度、准确度、线性和灵敏度方面对 3 种示范 mAb 药物进行了验证。该方法在临床样本中的成功应用证明了其在临床分析中的适用性。预计这种通用方法可以应用于其他基于 mAb 的药物,用于精准医学和临床研究。对于在三种不同浓度下测试的所有 mAb 药物,其误差均在 15% 以内。该通用方法在精密度、准确度、线性和灵敏度方面对 3 种示范 mAb 药物进行了验证。该方法在临床样本中的成功应用证明了其在临床分析中的适用性。预计这种通用方法可以应用于其他基于 mAb 的药物,用于精准医学和临床研究。对于在三种不同浓度下测试的所有 mAb 药物,其误差均在 15% 以内。该通用方法在精密度、准确度、线性和灵敏度方面对 3 种示范 mAb 药物进行了验证。该方法在临床样本中的成功应用证明了其在临床分析中的适用性。预计这种通用方法可以应用于其他基于 mAb 的药物,用于精准医学和临床研究。
更新日期:2018-08-01
中文翻译:

使用蛋白质 G 纯化结合使用 LC-MS/MS 的两个内标校准策略,开发用于量化人血浆中基于 IgG 的治疗性单克隆抗体的通用方法
近年来,单克隆抗体 (mAb) 药物在治疗各种疾病方面引起了广泛关注。免疫球蛋白 G (IgG) 在获得食品和药物管理局 (FDA) 批准的 mAb 药物中占很大比例。为了促进治疗药物监测和药代动力学/药效学研究,我们开发了一种通用的液相色谱-串联质谱 (LC-MS/MS) 方法来量化人血浆中基于 IgG 的 mAb 的浓度。选择了三种基于 IgG 的药物(贝伐单抗、纳武单抗和派姆单抗)来证明我们的方法。由于 G 蛋白珠具有捕获基于 IgG 的药物的普遍能力,因此它们被用于样品预处理。通过使用 LC-MS/MS 对胰蛋白酶消化后获得的替代肽进行定量。为了校准 LC-MS/MS 分析过程中发生的样品制备误差和基质效应,我们使用了两种内标 (IS) 方法,包括基于 IgG 的药物 IS 托珠单抗和柱后灌注 IS。发现使用两种内标可有效提高定量准确度,在三种不同浓度下测试的所有 mAb 药物的准确度均在 15% 以内。该通用方法在精密度、准确度、线性和灵敏度方面对 3 种示范 mAb 药物进行了验证。该方法在临床样本中的成功应用证明了其在临床分析中的适用性。预计这种通用方法可以应用于其他基于 mAb 的药物,用于精准医学和临床研究。我们使用了两种内标 (IS) 方法,包括基于 IgG 的药物 IS 托珠单抗和柱后灌注 IS。发现使用两种内标可有效提高定量准确度,在三种不同浓度下测试的所有 mAb 药物的准确度均在 15% 以内。该通用方法在精密度、准确度、线性和灵敏度方面对 3 种示范 mAb 药物进行了验证。该方法在临床样本中的成功应用证明了其在临床分析中的适用性。预计这种通用方法可以应用于其他基于 mAb 的药物,用于精准医学和临床研究。我们使用了两种内标 (IS) 方法,包括基于 IgG 的药物 IS 托珠单抗和柱后灌注 IS。发现使用两种内标可有效提高定量准确度,在三种不同浓度下测试的所有 mAb 药物的准确度均在 15% 以内。该通用方法在精密度、准确度、线性和灵敏度方面对 3 种示范 mAb 药物进行了验证。该方法在临床样本中的成功应用证明了其在临床分析中的适用性。预计这种通用方法可以应用于其他基于 mAb 的药物,用于精准医学和临床研究。对于在三种不同浓度下测试的所有 mAb 药物,其误差均在 15% 以内。该通用方法在精密度、准确度、线性和灵敏度方面对 3 种示范 mAb 药物进行了验证。该方法在临床样本中的成功应用证明了其在临床分析中的适用性。预计这种通用方法可以应用于其他基于 mAb 的药物,用于精准医学和临床研究。对于在三种不同浓度下测试的所有 mAb 药物,其误差均在 15% 以内。该通用方法在精密度、准确度、线性和灵敏度方面对 3 种示范 mAb 药物进行了验证。该方法在临床样本中的成功应用证明了其在临床分析中的适用性。预计这种通用方法可以应用于其他基于 mAb 的药物,用于精准医学和临床研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号