Journal of the Taiwan Institute of Chemical Engineers ( IF 5.5 ) Pub Date : 2018-02-21 , DOI: 10.1016/j.jtice.2018.01.021 Wenchao Zheng , Min Xiao , Helei Liu , Hongxia Gao , Zhiwu Liang
|
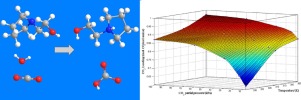
In this work, the equilibrium CO2 solubilities of the aqueous N-(2-hydroxyethyl) pyrrolidine (N-(2-HE) PRLD) at different concentrations (1.0 mol/L–5.0 mol/L) were measured at different temperatures (298 K–333 K) and pressures (8 kPa–101 kPa), using a vapor–liquid equilibrium apparatus. Four semi-empirical thermodynamic models (Cf model, Kent–Eisenberg model, Hu–Chakma model and Li–Shen model) were used to correlate and predict the CO2 solubility data of the N-(2-HE) PRLD-CO2–H2O system. The results showed that the Hu–Chakma model, which is one of the models that incorporates the effect of amine concentration and CO2 loading, and accounted for nonideality caused by higher temperature, species concentrations and CO2 loadings, was best able to predict results that are in agreement with the experimental vapor–liquid equilibrium measurements in this work.
中文翻译:

N-(2-羟乙基)吡咯烷水溶液中二氧化碳平衡溶解度的建模和实验
在这项工作中,在不同温度(1.0 mol / L–5.0 mol / L)下,测量了不同浓度(1.0 mol / L–5.0 mol / L)的N-(2-羟乙基)吡咯烷水溶液(N-(2-HE)PRLD)的平衡CO 2溶解度( 298 K–333 K)和压力(8 kPa–101 kPa),使用气液平衡仪。使用四个半经验热力学模型(C f模型,Kent-Eisenberg模型,Hu-Chakma模型和Li-Shen模型)来关联和预测N-(2-HE)PRLD-CO 2的CO 2溶解度数据–H 2 O系统。结果表明,Hu–Chakma模型是结合了胺浓度和CO 2影响的模型之一。负载,并考虑到较高的温度,物种浓度和CO 2负载导致的非理想性,最能够预测与这项工作中的实验气液平衡测量结果相符的结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号