当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Superlattice Formation of Crystal Water in Layered Double Hydroxides for Long‐Term and Fast Operation of Aqueous Rechargeable Batteries
Advanced Energy Materials ( IF 27.8 ) Pub Date : 2018-02-21 , DOI: 10.1002/aenm.201703572 Ji Hoon Lee 1 , Hyeon Jeong Lee 1 , Sun Hee Choi 2 , Jaeho Shin 3 , Sung-Yoon Chung 1 , Jang Wook Choi 3
Advanced Energy Materials ( IF 27.8 ) Pub Date : 2018-02-21 , DOI: 10.1002/aenm.201703572 Ji Hoon Lee 1 , Hyeon Jeong Lee 1 , Sun Hee Choi 2 , Jaeho Shin 3 , Sung-Yoon Chung 1 , Jang Wook Choi 3
Affiliation
|
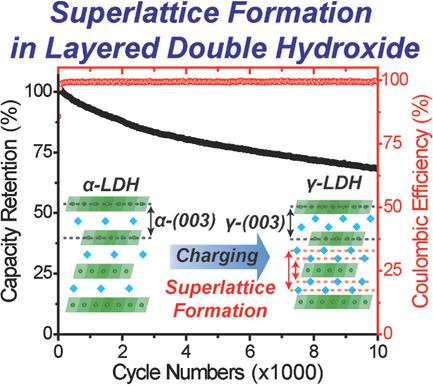
Aqueous rechargeable batteries (ARBs) are gaining increasing attention as alternatives to conventional nonaqueous lithium ion batteries. However, finding electrode materials with competitive electrochemical properties in various aspects is challenging. Moreover, the operation mechanism of some of high performance electrode materials is not fully understood. Here, an α‐phase layered double hydroxide (α‐LDH) working in alkaline electrolytes as an ARB cathode is reported. On charge, OH− carrier ions intercalate into the interlayer space and react with protons detached from the host structure to yield crystal water. This crystal water is then arranged in a superlattice during charging to accommodate carrier ions and stabilize the structure. The solid solution mixing of cobalt and nickel also stabilizes the structure during the wide range of redox swing of Ni from 2+ to 4+. In pairing with Fe3O4/Fe(OH)2 mixture, the α‐LDH exhibits 198.0 mA h g−1 at 3 A g−1, 68.3% capacity retention after 10 000 cycles, and 172.5 mA h g−1 at 1 min charge, demonstrating the promise of hydrated compounds for ARB electrodes. The present study elucidates that the arrangement of crystal water within the host framework plays a critical role in determining the electrochemical performance of the corresponding hydrated active compound in aqueous media.
中文翻译:

层状双氢氧化物中结晶水的超晶格形成,可长期且快速地运行可充电电池
作为传统的非水锂离子电池的替代品,水可充电电池(ARB)受到越来越多的关注。然而,寻找在各个方面具有竞争性电化学性能的电极材料是具有挑战性的。而且,尚未完全了解某些高性能电极材料的操作机理。在这里,报道了在碱性电解质中作为ARB阴极工作的α相层状双氢氧化物(α-LDH)。电荷,OH -载流子离子插入层间空间,并与从主体结构脱离的质子反应,生成结晶水。然后在充电过程中将这种结晶水排列在超晶格中,以容纳载流子离子并稳定结构。钴和镍的固溶体混合还可以使镍在从2+到4+的宽范围的氧化还原摆动期间稳定结构。与Fe 3 O 4 / Fe(OH)2混合物配对时,α-LDH在3 A g -1时表现出198.0 mA hg -1,在1万次循环后显示68.3%的容量保持率,在172.5 mA hg -1下显示每次充电1分钟,证明了ARB电极中水合化合物的前景。本研究阐明了在主体框架内结晶水的排列在确定相应的水合活性化合物在水性介质中的电化学性能方面起着至关重要的作用。
更新日期:2018-02-21
中文翻译:

层状双氢氧化物中结晶水的超晶格形成,可长期且快速地运行可充电电池
作为传统的非水锂离子电池的替代品,水可充电电池(ARB)受到越来越多的关注。然而,寻找在各个方面具有竞争性电化学性能的电极材料是具有挑战性的。而且,尚未完全了解某些高性能电极材料的操作机理。在这里,报道了在碱性电解质中作为ARB阴极工作的α相层状双氢氧化物(α-LDH)。电荷,OH -载流子离子插入层间空间,并与从主体结构脱离的质子反应,生成结晶水。然后在充电过程中将这种结晶水排列在超晶格中,以容纳载流子离子并稳定结构。钴和镍的固溶体混合还可以使镍在从2+到4+的宽范围的氧化还原摆动期间稳定结构。与Fe 3 O 4 / Fe(OH)2混合物配对时,α-LDH在3 A g -1时表现出198.0 mA hg -1,在1万次循环后显示68.3%的容量保持率,在172.5 mA hg -1下显示每次充电1分钟,证明了ARB电极中水合化合物的前景。本研究阐明了在主体框架内结晶水的排列在确定相应的水合活性化合物在水性介质中的电化学性能方面起着至关重要的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号