Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2018-02-21 , DOI: 10.1016/j.jaci.2017.12.1005 Yeon Duk Woo , Jaemoon Koh , Hye-Ryun Kang , Hye Young Kim , Doo Hyun Chung
|
Background
The chemokine X-C motif chemokine ligand 1 (XCL1)–X-C motif chemokine receptor 1 (XCR1) axis has been reported to play a role in immune homeostasis and inflammation. However, it is not known whether this axis has a critical function in patients with allergic asthma.
Objective
In the present study we explored whether the invariant natural killer T (iNKT) cell–mediated XCL1-XCR1 axis regulated allergic asthma.
Methods
Ovalbumin (OVA)– or house dust mite–induced asthma was developed in XCL1 or XCR1 knockout (KO) mice.
Results
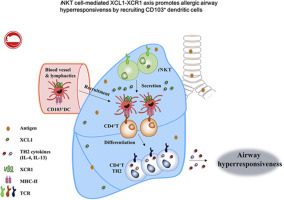
XCL1 or XCR1 KO mice showed attenuation in airway hyperresponsiveness (AHR), numbers of CD103+ dendritic cells (DCs), and TH2 responses in the lungs compared with wild-type (WT) mice during OVA- or house dust mite–induced asthma. These effects were reversed by intratracheal administration of recombinant XCL1 or adoptive transfer of CD103+ DCs but not CD11b+ DCs into XCL1 KO mice. Moreover, iNKT cells highly expressed XCL1 both in vitro and in vivo. On intranasal α-galactosyl ceramide challenge, CD103+ DC numbers in the lungs were increased in WT but not XCL1 KO mice. Furthermore, adoptive transfer of WT iNKT cells increased AHR, CD103+ DC recruitment, and TH2 responses in the lungs of CD1d KO mice during OVA-induced asthma, whereas adoptive transfer of XCL1-deficient iNKT cells did not. In human patients, percentages and XCL1 production capacity of iNKT cells from PBMCs were greater in patients with asthma than in healthy control subjects.
Conclusion
These data demonstrate that the iNKT cell–mediated XCL1-XCR1 axis promotes AHR by recruiting CD103+ DCs into the lung in patients with allergic asthma.
中文翻译:

不变的自然杀伤性T细胞介导的趋化因子XC基序趋化因子配体1–XC基序趋化因子受体1轴通过募集CD103 +树突状细胞促进过敏性气道高反应性
背景
据报道,趋化因子XC基序趋化因子配体1(XCL1)–XC基序趋化因子受体1(XCR1)轴在免疫稳态和炎症中起作用。但是,尚不清楚该轴在过敏性哮喘患者中是否具有关键功能。
客观的
在本研究中,我们探讨了不变的自然杀伤T(i NKT)细胞介导的XCL1-XCR1轴是否调节过敏性哮喘。
方法
在XCL1或XCR1基因敲除(KO)小鼠中出现了由卵白蛋白(OVA)或由屋尘螨引起的哮喘。
结果
XCL1或XCR1 KO小鼠中气道高反应性(AHR)显示衰减,CD103的号码+树突细胞(DC)和T ħ在肺部2周的回答与OVA-或屋尘中的野生型(WT)小鼠相比螨引起的哮喘。通过气管内施用重组XCL1或CD103 + DC而不是CD11b + DC的过继转移到XCL1 KO小鼠中,可以逆转这些作用。此外,i NKT细胞在体外和体内均高表达XCL1 。鼻内α-半乳糖基神经酰胺激发后,WT小鼠肺中的CD103 + DC数量增加,但XCL1 KO小鼠却没有。此外,WT i的代管转让NKT细胞增加AHR,CD103 + DC募集,和T ħ OVA诱导的哮喘期间的CD1d KO小鼠的肺中2个反应,而XCL1缺陷的过继转移我NKT细胞没有。在人类患者中,哮喘患者的PBMC i NKT细胞的百分比和XCL1产生能力要比健康对照组大。
结论
这些数据表明,i NKT细胞介导的XCL1-XCR1轴通过将CD103 + DC募集入过敏性哮喘患者的肺部来促进AHR 。









































 京公网安备 11010802027423号
京公网安备 11010802027423号