当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macrocycles Bearing Ferrocenyl Pendants and their Electrochemical Properties upon Binding to Divalent Transition Metal Cations.
ChemPlusChem ( IF 3.0 ) Pub Date : 2018-03-13 , DOI: 10.1002/cplu.201700550 Zhanghua Zeng 1 , Matthew J Belousoff 1 , Leone Spiccia 1 , Alan M Bond 1 , Angel A J Torriero 2
ChemPlusChem ( IF 3.0 ) Pub Date : 2018-03-13 , DOI: 10.1002/cplu.201700550 Zhanghua Zeng 1 , Matthew J Belousoff 1 , Leone Spiccia 1 , Alan M Bond 1 , Angel A J Torriero 2
Affiliation
|
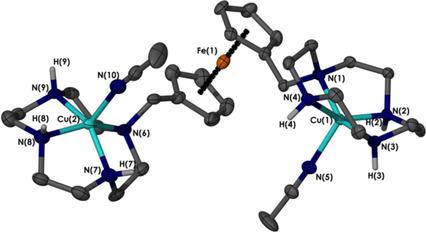
Metal complexes of Cu2+ , Co2+ , Cd2+ , Zn2+ , and Ni2+ formed with the ligands [Fc(cyclen)] (1) and [Fc(cyclen)2 ] (2) (Fc=ferrocene, cyclen=1,4,7,10-tetraazacyclododecane) are synthesised and characterised. The X-ray structure of the Cu2+ complex of 2, Fc([Cu(cyclen)(CH3 CN)]2 (ClO4 )4 , is reported, and shows that the two positively charged Cu2+ -cyclen units have a coordination number of five, adopting a distorted trigonal-bipyramidal configuration. The Cu2+ -cyclen units are arranged in a trans-like configuration with respect to the Fc group, presumably to minimise electrostatic repulsion. The voltammetric oxidation of the free ligands 1 and 2 in a CH2 Cl2 /CH3 CN (1:4) solvent mixture yields two closely spaced oxidation processes. Both electron-transfer steps are associated with the ferrocenyl moiety, implying strong communication between the cyclen nitrogen atoms and the ferrocenyl group. In contrast, cyclic voltammograms display only a simple reversible one-electron process if 1 and 2 are complexed with Cd2+ , Cu2+ , Zn2+ , Ni2+ , or Co2+ . Binding of these metal ions produces a significant shift in the reversible midpoint potential (Em ). Except for Ni2+ , Em is linearly proportional to the charge density of the transition metal ion, demonstrating that 1 and 2 may undergo redox switching. The diffusion coefficients of Fc, DmFc, 1 and 2, and their metal ion complexes correlate well with their molecular weights.
中文翻译:

带有二茂铁垂基的大环及其与二价过渡金属阳离子结合后的电化学性质。
与配体[Fc(cycln)](1)和[Fc(cycln)2](2)形成的Cu2 +,Co2 +,Cd2 +,Zn2 +和Ni2 +的金属配合物(Fc =二茂铁,cycln = 1、4、7,合成并表征了10-四氮杂环十二烷)。报告了2的Fc([Cu(cycln)(CH3 CN)] 2(ClO4)4的Cu2 +络合物的X射线结构,结果表明两个带正电的Cu2 +-环素单元的配位数为5 ,采用扭曲的三角-双锥体构型,相对于Fc基团,Cu2 +-环素单元呈反型排列,可能是为了最大程度地减少静电排斥力; CH2 Cl2 //中游离配体1和2的伏安氧化。 CH3 CN(1:4)溶剂混合物产生两个紧密间隔的氧化过程,两个电子转移步骤均与二茂铁基部分相关,暗示环氮原子和二茂铁基之间的强连通。相反,如果1和2与Cd2 +,Cu2 +,Zn2 +,Ni2 +或Co2 +络合,则循环伏安图仅显示简单的可逆单电子过程。这些金属离子的结合使可逆中点电势(Em)发生显着变化。除Ni2 +外,Em与过渡金属离子的电荷密度成线性比例,表明1和2可能会进行氧化还原转换。Fc,DmFc,1和2以及它们的金属离子配合物的扩散系数与其分子量密切相关。这些金属离子的结合使可逆中点电势(Em)发生显着变化。除Ni2 +外,Em与过渡金属离子的电荷密度成线性比例,表明1和2可能会进行氧化还原转换。Fc,DmFc,1和2以及它们的金属离子配合物的扩散系数与其分子量密切相关。这些金属离子的结合使可逆中点电势(Em)发生显着变化。除Ni2 +外,Em与过渡金属离子的电荷密度成线性比例,表明1和2可能会进行氧化还原转换。Fc,DmFc,1和2以及它们的金属离子配合物的扩散系数与其分子量密切相关。
更新日期:2018-03-13
中文翻译:

带有二茂铁垂基的大环及其与二价过渡金属阳离子结合后的电化学性质。
与配体[Fc(cycln)](1)和[Fc(cycln)2](2)形成的Cu2 +,Co2 +,Cd2 +,Zn2 +和Ni2 +的金属配合物(Fc =二茂铁,cycln = 1、4、7,合成并表征了10-四氮杂环十二烷)。报告了2的Fc([Cu(cycln)(CH3 CN)] 2(ClO4)4的Cu2 +络合物的X射线结构,结果表明两个带正电的Cu2 +-环素单元的配位数为5 ,采用扭曲的三角-双锥体构型,相对于Fc基团,Cu2 +-环素单元呈反型排列,可能是为了最大程度地减少静电排斥力; CH2 Cl2 //中游离配体1和2的伏安氧化。 CH3 CN(1:4)溶剂混合物产生两个紧密间隔的氧化过程,两个电子转移步骤均与二茂铁基部分相关,暗示环氮原子和二茂铁基之间的强连通。相反,如果1和2与Cd2 +,Cu2 +,Zn2 +,Ni2 +或Co2 +络合,则循环伏安图仅显示简单的可逆单电子过程。这些金属离子的结合使可逆中点电势(Em)发生显着变化。除Ni2 +外,Em与过渡金属离子的电荷密度成线性比例,表明1和2可能会进行氧化还原转换。Fc,DmFc,1和2以及它们的金属离子配合物的扩散系数与其分子量密切相关。这些金属离子的结合使可逆中点电势(Em)发生显着变化。除Ni2 +外,Em与过渡金属离子的电荷密度成线性比例,表明1和2可能会进行氧化还原转换。Fc,DmFc,1和2以及它们的金属离子配合物的扩散系数与其分子量密切相关。这些金属离子的结合使可逆中点电势(Em)发生显着变化。除Ni2 +外,Em与过渡金属离子的电荷密度成线性比例,表明1和2可能会进行氧化还原转换。Fc,DmFc,1和2以及它们的金属离子配合物的扩散系数与其分子量密切相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号