当前位置:
X-MOL 学术
›
Mater. Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An alternative route to single ion conductivity using multi-ionic salts†
Materials Horizons ( IF 13.3 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1039/c7mh01130j Sumanth Chereddy 1, 2, 3, 4 , Parameswara Rao Chinnam 1, 2, 3, 4 , Vijay Chatare 1, 2, 3, 4 , Stephen Patrick diLuzio 1, 2, 3, 4 , Mallory P. Gobet 4, 5, 6, 7 , Steven G. Greenbaum 4, 5, 6, 7 , Stephanie L. Wunder 4, 5, 6, 7
Materials Horizons ( IF 13.3 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1039/c7mh01130j Sumanth Chereddy 1, 2, 3, 4 , Parameswara Rao Chinnam 1, 2, 3, 4 , Vijay Chatare 1, 2, 3, 4 , Stephen Patrick diLuzio 1, 2, 3, 4 , Mallory P. Gobet 4, 5, 6, 7 , Steven G. Greenbaum 4, 5, 6, 7 , Stephanie L. Wunder 4, 5, 6, 7
Affiliation
|
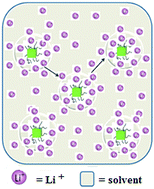
Multi-ionic lithium salts comprised of polyoligomeric silsesquioxanes (POSS) functionalized with eight – (LiNSO2CF3) groups, referred to as POSS-(LiNSO2CF3)8, can be dissolved at very high loadings into tetraglyme (G4), where they can be considered solvent-in-salt electrolytes. With increasing dilution, colloidal solutions are formed. Two systems were investigated, neat POSS-(LiNSO2CF3)8 in G4 and mixtures of POSS-(LiNSO2CF3)8 with LiPF6 or lithium bis(trifluoromethanesulfonyl)imide (LiTFSI). PFG-NMR indicates that Li can be un-dissociated, completely dissociated and surrounded by G4 molecules, or as contact ion pairs (in which there are 3–4 ether oxygen contacts and one contact with the oxygen from the anion). Equilibria exist between the species of POSS-(LiNSO2CF3)8 and if there is rapid equilibration between the Li states, and close enough proximity between the POSS-(LiNSO2CF3), then the Li+ ions can migrate by a Grotthus-type coordinated hopping mechanism, as well as by a purely diffusive motion. Unlike polymer single ion conductors, where the backbone flexibility permits cluster/aggregate formation, which inhibits escape and mobility of the Li+ ions, the rigid POSS cube and its colloidal structure in G4 prevents formation of POSS-(NSO2CF3−)⋯Li+⋯(−CF3NSO2)-POSS triplets. Instead, the solvated Li+ in POSS-(NSO2CF3−)⋯Li+⋯G4 can be more easily removed to form conductive G4⋯Li+−⋯G4. Good ionic conductivities (∼10−4 S cm−1) and lithium ion transference numbers of tPP+ = 0.65 can be achieved in these systems. Mixtures of 80 wt% LiTFSI and 20 wt% POSS-(LiNSO2CF3)8 in G4 at an O/Li ratio of 20/1, yield both high conductivity (σ = 3.3 × 10−3 S cm−1) and high (tPP+ = 0.65) transference number. Stable cycling between C/2 and 2C with high capacity retention was achieved using Li/[G4/80 wt% LiTFSI/20 wt% POSS-(LiNSO2CF3)8]/LiFePO4 half-cells.
中文翻译:

使用多离子盐实现单离子电导率的另一种途径†
包含POSS-(LiNSO 2 CF 3)8的,由八个(LiNSO 2 CF 3)基团官能化的低聚倍半硅氧烷(POSS)组成的多离子锂盐,可以以很高的负载量溶解成四甘醇二甲醚(G 4)。 ,可以将其视为盐中溶剂型电解质。随着稀释度的增加,形成胶体溶液。研究了两个系统:纯净的POSS-(LiNSO 2 CF 3)8在G 4中和POSS-(LiNSO 2 CF 3)8与LiPF 6的混合物或双(三氟甲磺酰基)酰亚胺锂(LiTFSI)。PFG-NMR表明,Li可以未解离,完全解离并被G 4分子包围,或作为接触离子对(其中有3–4个醚氧接触并且一个与阴离子中的氧接触)。在POSS-(LiNSO 2 CF 3)8的物种之间存在平衡,如果在Li状态之间存在快速平衡,并且在POSS-(LiNSO 2 CF 3)之间足够接近,则Li +离子可以通过Grotthus型协调跳跃机制以及纯扩散运动迁移。不同于聚合物单离子导体,其中所述主链的灵活性允许群集/聚集体形成,其抑制逸出和锂的迁移率+离子,G中的刚性POSS立方体及其胶体结构4防止形成POSS-的(NSO 2 CF 3 - )⋯Li + ⋯(− CF 3 NSO 2)-POSS三胞胎。取而代之的是,溶剂化的锂+在POSS-(NSO 2 CF 3 -)⋯栗+ ⋯ģ 4可以更容易地除去以形成导电性G 4 ⋯Li + − ⋯G 4。在这些系统中,可以获得良好的离子电导率(〜10 -4 S cm -1)和锂离子转移数t PP + = 0.65。以20/1的O / Li比在G 4中以80 wt%的LiTFSI和20 wt%的POSS-(LiNSO 2 CF 3)8的混合物产生高电导率(σ = 3.3×10 -3 S cm -1)高(t PP += 0.65)转移编号。使用Li / [G达到C / 2和2C之间稳定的循环具有高容量保持4 /80重量%的LiTFSI / 20重量%POSS-(林索2 CF 3)8 ] /的LiFePO 4的半电池。
更新日期:2018-02-20
中文翻译:

使用多离子盐实现单离子电导率的另一种途径†
包含POSS-(LiNSO 2 CF 3)8的,由八个(LiNSO 2 CF 3)基团官能化的低聚倍半硅氧烷(POSS)组成的多离子锂盐,可以以很高的负载量溶解成四甘醇二甲醚(G 4)。 ,可以将其视为盐中溶剂型电解质。随着稀释度的增加,形成胶体溶液。研究了两个系统:纯净的POSS-(LiNSO 2 CF 3)8在G 4中和POSS-(LiNSO 2 CF 3)8与LiPF 6的混合物或双(三氟甲磺酰基)酰亚胺锂(LiTFSI)。PFG-NMR表明,Li可以未解离,完全解离并被G 4分子包围,或作为接触离子对(其中有3–4个醚氧接触并且一个与阴离子中的氧接触)。在POSS-(LiNSO 2 CF 3)8的物种之间存在平衡,如果在Li状态之间存在快速平衡,并且在POSS-(LiNSO 2 CF 3)之间足够接近,则Li +离子可以通过Grotthus型协调跳跃机制以及纯扩散运动迁移。不同于聚合物单离子导体,其中所述主链的灵活性允许群集/聚集体形成,其抑制逸出和锂的迁移率+离子,G中的刚性POSS立方体及其胶体结构4防止形成POSS-的(NSO 2 CF 3 - )⋯Li + ⋯(− CF 3 NSO 2)-POSS三胞胎。取而代之的是,溶剂化的锂+在POSS-(NSO 2 CF 3 -)⋯栗+ ⋯ģ 4可以更容易地除去以形成导电性G 4 ⋯Li + − ⋯G 4。在这些系统中,可以获得良好的离子电导率(〜10 -4 S cm -1)和锂离子转移数t PP + = 0.65。以20/1的O / Li比在G 4中以80 wt%的LiTFSI和20 wt%的POSS-(LiNSO 2 CF 3)8的混合物产生高电导率(σ = 3.3×10 -3 S cm -1)高(t PP += 0.65)转移编号。使用Li / [G达到C / 2和2C之间稳定的循环具有高容量保持4 /80重量%的LiTFSI / 20重量%POSS-(林索2 CF 3)8 ] /的LiFePO 4的半电池。



























 京公网安备 11010802027423号
京公网安备 11010802027423号