当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon Incorporation and Anion Dynamics as Synergistic Drivers for Ultrafast Diffusion in Superionic LiCB11H12 and NaCB11H12
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2018-02-20 , DOI: 10.1002/aenm.201703422 Mirjana Dimitrievska 1, 2 , Patrick Shea 3 , Kyoung E. Kweon 3 , Marnik Bercx 4 , Joel B. Varley 3 , Wan Si Tang 2, 5 , Alexander V. Skripov 6 , Vitalie Stavila 7 , Terrence J. Udovic 2 , Brandon C. Wood 3
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2018-02-20 , DOI: 10.1002/aenm.201703422 Mirjana Dimitrievska 1, 2 , Patrick Shea 3 , Kyoung E. Kweon 3 , Marnik Bercx 4 , Joel B. Varley 3 , Wan Si Tang 2, 5 , Alexander V. Skripov 6 , Vitalie Stavila 7 , Terrence J. Udovic 2 , Brandon C. Wood 3
Affiliation
|
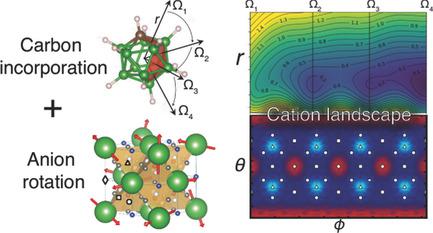
The disordered phases of LiCB11H12 and NaCB11H12 possess superb superionic conductivities that make them suitable as solid electrolytes. In these materials, cation diffusion correlates with high orientational mobilities of the CB11H12− anions; however, the precise relationship has yet to be demonstrated. In this work, ab initio molecular dynamics and quasielastic neutron scattering are combined to probe anion reorientations and their mechanistic connection to cation mobility over a range of timescales and temperatures. It is found that anions do not rotate freely, but rather transition rapidly between orientations defined by the cation sublattice symmetry. The symmetry‐breaking carbon atom in CB11H12− also plays a critical role by perturbing the energy landscape along the instantaneous orientation of the anion dipole, which couples fluctuations in the cation probability density directly to the anion motion. Anion reorientation rates exceed 3 × 1010 s−1, suggesting the underlying energy landscape fluctuates dynamically on diffusion‐relevant timescales. Furthermore, carbon is found to modify the orientational preferences of the anions and aid rotational mobility, creating additional symmetry incompatibilities that inhibit ordering. The results suggest that synergy between the anion reorientational dynamics and the carbon‐modified cation–anion interaction accounts for the higher ionic conductivity in CB11H12− salts compared with B12H122−.
中文翻译:

碳结合和阴离子动力学作为超离子LiCB11H12和NaCB11H12中超快扩散的协同驱动因素
LiCB 11 H 12和NaCB 11 H 12的无序相具有极好的超离子电导率,使其适合用作固体电解质。在这些材料中,阳离子扩散相关因素与CB的高迁移率取向11 ħ 12 -阴离子 但是,确切的关系尚待证明。在这项工作中,从头算分子动力学和准弹性中子散射相结合,以探测阴离子的重新定向及其在一定时间范围和温度范围内与阳离子迁移率的机理联系。发现阴离子不能自由旋转,而是在由阳离子亚晶格对称性定义的取向之间快速转变。在CB对称破碳原子11 ħ 12 -还通过沿着阴离子偶极子,瞬时取向扰动能量景观中起关键作用,其直接在阳离子概率密度至阴离子运动耦合的波动。阴离子再取向速率超过3×10 10 s -1,表明潜在的能源格局在与扩散相关的时标上动态波动。此外,发现碳可改变阴离子的取向偏好并有助于旋转迁移率,从而产生其他抑制对称性的不对称性。结果表明阴离子重取向动力学和碳改性的阳离子-阴离子相互作用之间的协同作用占在CB较高的离子电导率11 ħ 12 -盐与乙相比12 ħ 12 2-。
更新日期:2018-02-20
中文翻译:

碳结合和阴离子动力学作为超离子LiCB11H12和NaCB11H12中超快扩散的协同驱动因素
LiCB 11 H 12和NaCB 11 H 12的无序相具有极好的超离子电导率,使其适合用作固体电解质。在这些材料中,阳离子扩散相关因素与CB的高迁移率取向11 ħ 12 -阴离子 但是,确切的关系尚待证明。在这项工作中,从头算分子动力学和准弹性中子散射相结合,以探测阴离子的重新定向及其在一定时间范围和温度范围内与阳离子迁移率的机理联系。发现阴离子不能自由旋转,而是在由阳离子亚晶格对称性定义的取向之间快速转变。在CB对称破碳原子11 ħ 12 -还通过沿着阴离子偶极子,瞬时取向扰动能量景观中起关键作用,其直接在阳离子概率密度至阴离子运动耦合的波动。阴离子再取向速率超过3×10 10 s -1,表明潜在的能源格局在与扩散相关的时标上动态波动。此外,发现碳可改变阴离子的取向偏好并有助于旋转迁移率,从而产生其他抑制对称性的不对称性。结果表明阴离子重取向动力学和碳改性的阳离子-阴离子相互作用之间的协同作用占在CB较高的离子电导率11 ħ 12 -盐与乙相比12 ħ 12 2-。











































 京公网安备 11010802027423号
京公网安备 11010802027423号