当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into the Activation of Soluble Guanylate Cyclase by Carbon Monoxide: A Multistep Mechanism Proposed for the BAY 41-2272 Induced Formation of 5-Coordinate CO–Heme
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1021/acs.biochem.7b01240 Ryu Makino 1 , Yuji Obata 1 , Motonari Tsubaki 2 , Tetsutaro Iizuka 3 , Yuki Hamajima 1 , Yasuyuki Kato-Yamada 1 , Keisuke Mashima 1 , Yoshitsugu Shiro 4
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1021/acs.biochem.7b01240 Ryu Makino 1 , Yuji Obata 1 , Motonari Tsubaki 2 , Tetsutaro Iizuka 3 , Yuki Hamajima 1 , Yasuyuki Kato-Yamada 1 , Keisuke Mashima 1 , Yoshitsugu Shiro 4
Affiliation
|
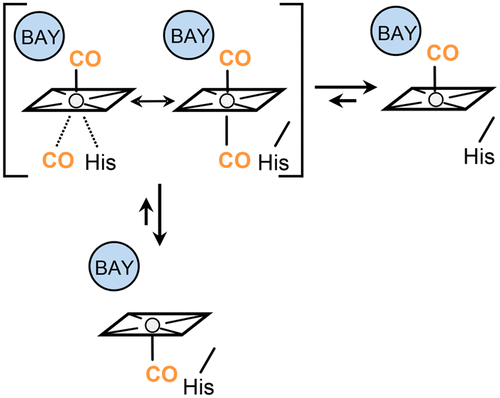
Soluble guanylate cyclase (sGC) is a heme-containing enzyme that catalyzes cGMP production upon sensing NO. While the CO adduct, sGC–CO, is much less active, the allosteric regulator BAY 41-2272 stimulates the cGMP productivity to the same extent as that of sGC–NO. The stimulatory effect has been thought to be likely associated with Fe-His bond cleavage leading to 5-coordinate CO–heme, but the detailed mechanism remains unresolved. In this study, we examined the mechanism under the condition including BAY 41-2272, 2′-deoxy-3′-GMP and foscarnet. The addition of these effectors caused the original 6-coordinate CO–heme to convert to an end product that was an equimolar mixture of a 5- and a new 6-coordinate CO–heme, as assessed by IR spectral measurements. The two types of CO–hemes in the end product were further confirmed by CO dissociation kinetics. Stopped-flow measurements under the condition indicated that the ferrous sGC bound CO as two reversible steps, where the primary step was assigned to the full conversion of the ferrous enzyme to the 6-coordinate CO–heme, and subsequently followed by the slower second step leading a partial conversion of the 6-coordinate CO–heme to the 5-coordinate CO–heme. The observed rates for both steps linearly depended on CO concentrations. The unexpected CO dependence of the rates in the second step supports a multistep mechanism, in which the 5-coordinate CO–heme is led by CO release from a putative bis-carbonyl intermediate that is likely provided by the binding of a second CO to the 6-coordinate CO–heme. This mechanism provides a new aspect on the activation of sGC by CO.
中文翻译:

一氧化碳活化可溶性鸟苷酸环化酶的机理研究:BAY 41-2272诱导形成5坐标CO-血红素的多步机制
可溶性鸟苷酸环化酶(sGC)是一种含血红素的酶,可在感应到NO时催化cGMP的产生。尽管一氧化碳加合物sGC-CO的活性低得多,但变构调节剂BAY 41-2272刺激cGMP生产力的程度与sGC-NO相同。人们认为这种刺激作用可能与Fe-His键断裂导致5坐标的CO-血红素有关,但具体的机制仍未解决。在这项研究中,我们研究了在包括BAY 41-2272、2'-deoxy-3'-GMP和膦甲酸在内的条件下的作用机理。这些效应物的加入导致原始的6坐标CO-血红素转化为最终产物,最终产物是5和新的6坐标CO-血红素的等摩尔混合物,通过红外光谱测量得到评估。最终产物中的两种类型的CO-hemes通过CO解离动力学得到了进一步的证实。在这种情况下的停止流量测量表明,sGC结合亚铁是两个可逆的步骤,其中第一步是将亚铁酶完全转化为6坐标的CO-血红素,随后是较慢的第二步导致将6坐标的CO-血红素部分转化为5坐标的CO-血红素。观察到的两个步骤的速率均线性依赖于CO浓度。在第二步中,CO对速率的出乎意料的依赖性支持了一个多步机制,其中五坐标的CO-血红素是由假定的双羰基中间体释放出的CO导致的,这可能是第二种CO与C的结合所导致的。 6坐标CO-血红素。此机制为CO激活sGC提供了一个新的方面。
更新日期:2018-02-20
中文翻译:

一氧化碳活化可溶性鸟苷酸环化酶的机理研究:BAY 41-2272诱导形成5坐标CO-血红素的多步机制
可溶性鸟苷酸环化酶(sGC)是一种含血红素的酶,可在感应到NO时催化cGMP的产生。尽管一氧化碳加合物sGC-CO的活性低得多,但变构调节剂BAY 41-2272刺激cGMP生产力的程度与sGC-NO相同。人们认为这种刺激作用可能与Fe-His键断裂导致5坐标的CO-血红素有关,但具体的机制仍未解决。在这项研究中,我们研究了在包括BAY 41-2272、2'-deoxy-3'-GMP和膦甲酸在内的条件下的作用机理。这些效应物的加入导致原始的6坐标CO-血红素转化为最终产物,最终产物是5和新的6坐标CO-血红素的等摩尔混合物,通过红外光谱测量得到评估。最终产物中的两种类型的CO-hemes通过CO解离动力学得到了进一步的证实。在这种情况下的停止流量测量表明,sGC结合亚铁是两个可逆的步骤,其中第一步是将亚铁酶完全转化为6坐标的CO-血红素,随后是较慢的第二步导致将6坐标的CO-血红素部分转化为5坐标的CO-血红素。观察到的两个步骤的速率均线性依赖于CO浓度。在第二步中,CO对速率的出乎意料的依赖性支持了一个多步机制,其中五坐标的CO-血红素是由假定的双羰基中间体释放出的CO导致的,这可能是第二种CO与C的结合所导致的。 6坐标CO-血红素。此机制为CO激活sGC提供了一个新的方面。











































 京公网安备 11010802027423号
京公网安备 11010802027423号