当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unique Biochemical and Sequence Features Enable BluB To Destroy Flavin and Distinguish BluB from the Flavin Monooxygenase Superfamily
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acs.biochem.7b01193 Amrita B. Hazra 1, 2 , David P. Ballou 3 , Michiko E. Taga 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acs.biochem.7b01193 Amrita B. Hazra 1, 2 , David P. Ballou 3 , Michiko E. Taga 1
Affiliation
|
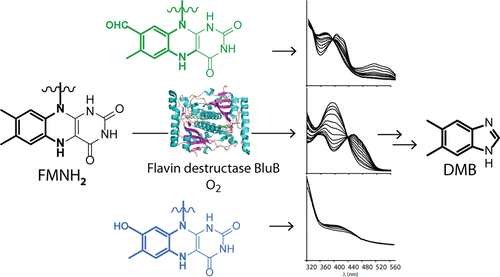
Vitamin B12 (cobalamin) is an essential micronutrient for humans that is synthesized by only a subset of bacteria and archaea. The aerobic biosynthesis of 5,6-dimethylbenzimidazole, the lower axial ligand of cobalamin, is catalyzed by the “flavin destructase” enzyme BluB, which fragments reduced flavin mononucleotide following its reaction with oxygen to yield this ligand. BluB is similar in sequence and structure to members of the flavin oxidoreductase superfamily, yet the flavin destruction process has remained elusive. Using stopped-flow spectrophotometry, we find that the flavin destructase reaction of BluB from Sinorhizobium meliloti is initiated with canonical flavin–O2 chemistry. A C4a-peroxyflavin intermediate is rapidly formed in BluB upon reaction with O2, and has properties similar to those of flavin-dependent hydroxylases. Analysis of reaction mixtures containing flavin analogues indicates that both formation of the C4a-peroxyflavin and the subsequent destruction of the flavin to form 5,6-dimethylbenzimidazole are influenced by the electronic properties of the flavin isoalloxazine ring. The flavin destruction phase of the reaction, which results from the decay of the C4a-peroxyflavin intermediate, occurs more efficiently at pH >7.5. Furthermore, the BluB mutants D32N and S167G are specifically impaired in the flavin destruction phase of the reaction; nevertheless, both form the C4a-peroxyflavin nearly quantitatively. Coupled with a phylogenetic analysis of BluB and related flavin-dependent enzymes, these results demonstrate that the BluB flavin destructase family can be identified by the presence of active site residues D32 and S167.
中文翻译:

独特的生化和序列特征使BluB能够从Flavin单加氧酶超家族中破坏Flavin并区分BluB。
维生素B 12(钴胺素)是人类必需的微量营养素,仅由一部分细菌和古细菌合成。钴胺素的较低轴向配体5,6-二甲基苯并咪唑的有氧生物合成是由“黄素破坏酶” BluB催化的,该酶在与氧气反应后还原还原的黄素单核苷酸以产生该配体。BluB的序列和结构与黄素氧化还原酶超家族成员相似,但黄素破坏过程仍然难以捉摸。使用停止流式分光光度法,我们发现从标准的黄素-O 2引发了苜蓿中华根瘤菌的BluB的黄素破坏酶反应化学。与O 2反应后,BluB中会迅速形成C4a-过氧黄素中间体,其性质类似于黄素依赖性羟化酶。含有黄素类似物的反应混合物的分析表明,C4a-过氧黄素的形成以及黄素随后的破坏以形成5,6-二甲基苯并咪唑都受到黄素异四恶嗪环的电子性质的影响。由C4a-过氧黄素中间体的降解导致的反应黄素破坏相在pH> 7.5时更有效地发生。此外,在反应的黄素破坏阶段,BluB突变体D32N和S167G受到特别的损害。然而,两者几乎都定量形成了C4a-过氧黄素。结合BluB和相关的黄素依赖性酶的系统发育分析,
更新日期:2018-02-19
中文翻译:

独特的生化和序列特征使BluB能够从Flavin单加氧酶超家族中破坏Flavin并区分BluB。
维生素B 12(钴胺素)是人类必需的微量营养素,仅由一部分细菌和古细菌合成。钴胺素的较低轴向配体5,6-二甲基苯并咪唑的有氧生物合成是由“黄素破坏酶” BluB催化的,该酶在与氧气反应后还原还原的黄素单核苷酸以产生该配体。BluB的序列和结构与黄素氧化还原酶超家族成员相似,但黄素破坏过程仍然难以捉摸。使用停止流式分光光度法,我们发现从标准的黄素-O 2引发了苜蓿中华根瘤菌的BluB的黄素破坏酶反应化学。与O 2反应后,BluB中会迅速形成C4a-过氧黄素中间体,其性质类似于黄素依赖性羟化酶。含有黄素类似物的反应混合物的分析表明,C4a-过氧黄素的形成以及黄素随后的破坏以形成5,6-二甲基苯并咪唑都受到黄素异四恶嗪环的电子性质的影响。由C4a-过氧黄素中间体的降解导致的反应黄素破坏相在pH> 7.5时更有效地发生。此外,在反应的黄素破坏阶段,BluB突变体D32N和S167G受到特别的损害。然而,两者几乎都定量形成了C4a-过氧黄素。结合BluB和相关的黄素依赖性酶的系统发育分析,











































 京公网安备 11010802027423号
京公网安备 11010802027423号