当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thienyl-Substituted α-Ketoamide: A Less Hydrophobic Reactive Group for Photo-Affinity Labeling
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acschembio.7b00988 Eisuke Ota 1, 2 , Kazuteru Usui 3 , Kana Oonuma 4 , Hiroyuki Koshino 4 , Shigeru Nishiyama 2 , Go Hirai 1, 3, 4 , Mikiko Sodeoka 1, 4, 5
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acschembio.7b00988 Eisuke Ota 1, 2 , Kazuteru Usui 3 , Kana Oonuma 4 , Hiroyuki Koshino 4 , Shigeru Nishiyama 2 , Go Hirai 1, 3, 4 , Mikiko Sodeoka 1, 4, 5
Affiliation
|
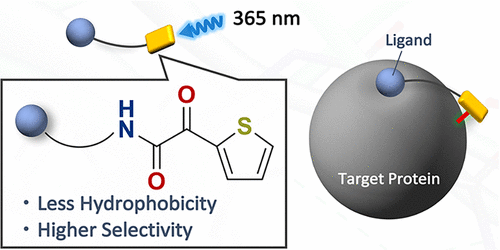
Photoaffinity labeling (PAL) is an important tool in chemical biology research, but application of α-ketoamides for PAL has been hampered by their photoinstability. Here, we show that 2-thienyl-substituted α-ketoamide is a superior photoreactive group for PAL. Studies with a series of synthetic mannose-conjugated α-ketoamides revealed that 2-thienyl substitution of α-ketoamide decreased the electrophilicity of the keto group and reduced the rate of photodegradation. Mannose-conjugated thienyl α-ketoamide showed greater concanavalin A labeling efficiency than other alkyl or phenyl-substituted α-ketoamides. In comparison with representative conventional photoreactive groups, 2-thienyl ketoamide showed reduced labeling of nontarget proteins, probably owing to its lower hydrophobicity.
中文翻译:

噻吩基取代的α-酮酰胺:较少的疏水性反应基团,用于光亲和标记
光亲和标记(PAL)是化学生物学研究中的重要工具,但是α-酮酰胺在PAL中的应用由于其光不稳定性而受到阻碍。在这里,我们表明2-噻吩基取代的α-酮酰胺是PAL的优良光反应性基团。对一系列合成甘露糖缀合的α-酮酰胺的研究表明,α-酮酰胺的2-噻吩基取代会降低酮基的亲电性,并降低光降解速率。甘露糖缀合的噻吩基α-酮酰胺比其他烷基或苯基取代的α-酮酰胺显示更高的伴刀豆球蛋白A标记效率。与代表性的常规光反应性基团相比,2-噻吩基酮酰胺的非靶蛋白标记减少,可能是由于其较低的疏水性。
更新日期:2018-02-19
中文翻译:

噻吩基取代的α-酮酰胺:较少的疏水性反应基团,用于光亲和标记
光亲和标记(PAL)是化学生物学研究中的重要工具,但是α-酮酰胺在PAL中的应用由于其光不稳定性而受到阻碍。在这里,我们表明2-噻吩基取代的α-酮酰胺是PAL的优良光反应性基团。对一系列合成甘露糖缀合的α-酮酰胺的研究表明,α-酮酰胺的2-噻吩基取代会降低酮基的亲电性,并降低光降解速率。甘露糖缀合的噻吩基α-酮酰胺比其他烷基或苯基取代的α-酮酰胺显示更高的伴刀豆球蛋白A标记效率。与代表性的常规光反应性基团相比,2-噻吩基酮酰胺的非靶蛋白标记减少,可能是由于其较低的疏水性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号