Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Haloperidol on Survival Among Critically Ill Adults With a High Risk of Delirium
JAMA ( IF 63.1 ) Pub Date : 2018-02-20 , DOI: 10.1001/jama.2018.0160 Mark van den Boogaard 1 , Arjen J C Slooter 2 , Roger J M Brüggemann 3 , Lisette Schoonhoven 4, 5, 6 , Albertus Beishuizen 7 , J Wytze Vermeijden 7 , Danie Pretorius 8 , Jan de Koning 9 , Koen S Simons 10 , Paul J W Dennesen 11 , Peter H J Van der Voort 12, 13 , Saskia Houterman 14 , J G van der Hoeven 1 , Peter Pickkers 1 , , Margaretha C. E. van der Woude 15 , Anna Besselink 16 , Lieuwe S Hofstra 17 , Peter E Spronk 18 , Walter van den Bergh 19 , Dirk W Donker 2 , Malaika Fuchs 20 , Attila Karakus 21 , M Koeman 22 , Mirella van Duijnhoven 23 , Gerjon Hannink 24
JAMA ( IF 63.1 ) Pub Date : 2018-02-20 , DOI: 10.1001/jama.2018.0160 Mark van den Boogaard 1 , Arjen J C Slooter 2 , Roger J M Brüggemann 3 , Lisette Schoonhoven 4, 5, 6 , Albertus Beishuizen 7 , J Wytze Vermeijden 7 , Danie Pretorius 8 , Jan de Koning 9 , Koen S Simons 10 , Paul J W Dennesen 11 , Peter H J Van der Voort 12, 13 , Saskia Houterman 14 , J G van der Hoeven 1 , Peter Pickkers 1 , , Margaretha C. E. van der Woude 15 , Anna Besselink 16 , Lieuwe S Hofstra 17 , Peter E Spronk 18 , Walter van den Bergh 19 , Dirk W Donker 2 , Malaika Fuchs 20 , Attila Karakus 21 , M Koeman 22 , Mirella van Duijnhoven 23 , Gerjon Hannink 24
Affiliation
|
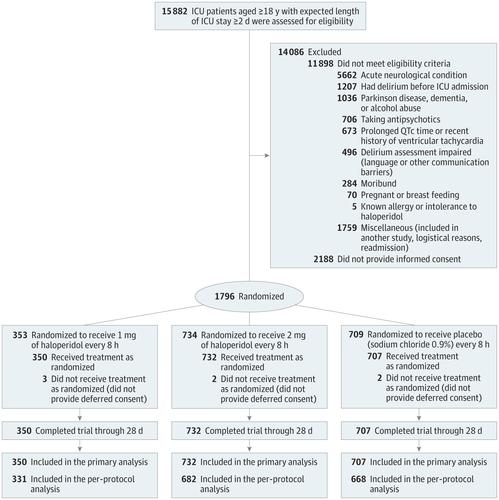
Importance Results of studies on use of prophylactic haloperidol in critically ill adults are inconclusive, especially in patients at high risk of delirium. Objective To determine whether prophylactic use of haloperidol improves survival among critically ill adults at high risk of delirium, which was defined as an anticipated intensive care unit (ICU) stay of at least 2 days. Design, Setting, and Participants Randomized, double-blind, placebo-controlled investigator-driven study involving 1789 critically ill adults treated at 21 ICUs, at which nonpharmacological interventions for delirium prevention are routinely used in the Netherlands. Patients without delirium whose expected ICU stay was at least a day were included. Recruitment was from July 2013 to December 2016 and follow-up was conducted at 90 days with the final follow-up on March 1, 2017. Interventions Patients received prophylactic treatment 3 times daily intravenously either 1 mg (n = 350) or 2 mg (n = 732) of haloperidol or placebo (n = 707), consisting of 0.9% sodium chloride. Main Outcome and Measures The primary outcome was the number of days that patients survived in 28 days. There were 15 secondary outcomes, including delirium incidence, 28-day delirium-free and coma-free days, duration of mechanical ventilation, and ICU and hospital length of stay. Results All 1789 randomized patients (mean, age 66.6 years [SD, 12.6]; 1099 men [61.4%]) completed the study. The 1-mg haloperidol group was prematurely stopped because of futility. There was no difference in the median days patients survived in 28 days, 28 days in the 2-mg haloperidol group vs 28 days in the placebo group, for a difference of 0 days (95% CI, 0-0; P = .93) and a hazard ratio of 1.003 (95% CI, 0.78-1.30, P=.82). All of the 15 secondary outcomes were not statistically different. These included delirium incidence (mean difference, 1.5%, 95% CI, −3.6% to 6.7%), delirium-free and coma-free days (mean difference, 0 days, 95% CI, 0-0 days), and duration of mechanical ventilation, ICU, and hospital length of stay (mean difference, 0 days, 95% CI, 0-0 days for all 3 measures). The number of reported adverse effects did not differ between groups (2 [0.3%] for the 2-mg haloperidol group vs 1 [0.1%] for the placebo group). Conclusions and Relevance Among critically ill adults at high risk of delirium, the use of prophylactic haloperidol compared with placebo did not improve survival at 28 days. These findings do not support the use of prophylactic haloperidol for reducing mortality in critically ill adults. Trial Registration clinicaltrials.gov Identifier: NCT01785290
中文翻译:

氟哌啶醇对谵妄高危危重患者生存率的影响
重要性 关于在危重成人患者中预防性使用氟哌啶醇的研究结果尚无定论,尤其是在谵妄高危患者中。目的 确定预防性使用氟哌啶醇是否能改善谵妄高危危重成人患者的生存率,谵妄的定义是预计在重症监护室 (ICU) 停留至少 2 天。设计、设置和参与者 随机、双盲、安慰剂对照、研究者驱动的研究,涉及 1789 名在 21 个 ICU 接受治疗的危重成人,在这些 ICU 中,荷兰常规使用预防谵妄的非药物干预措施。预计入住ICU至少一天的没有谵妄的患者被包括在内。招募时间为2013年7月至2016年12月,随访90天,末次随访于2017年3月1日。干预 患者每天接受 3 次静脉内预防性治疗,即 1 毫克(n = 350)或 2 毫克(n = 732)氟哌啶醇或安慰剂(n = 707),由 0.9% 氯化钠组成。主要结果和措施主要结果是患者在 28 天内存活的天数。有 15 个次要结果,包括谵妄发生率、28 天无谵妄和昏迷天数、机械通气持续时间以及 ICU 和住院时间。结果 所有 1789 名随机患者(平均年龄 66.6 岁 [SD,12.6];1099 名男性 [61.4%])完成了研究。1mg 氟哌啶醇组因无效而提前停止。患者在 28 天内存活的中位天数没有差异,2 毫克氟哌啶醇组为 28 天,安慰剂组为 28 天,差异为 0 天(95% CI,0-0;P = . 93) 和 1.003 的风险比 (95% CI, 0.78-1.30, P=.82)。所有 15 项次要结果均无统计学差异。这些包括谵妄发生率(平均差异,1.5%,95% CI,-3.6% 至 6.7%),无谵妄和无昏迷天数(平均差异,0 天,95% CI,0-0 天)和持续时间机械通气、ICU 和住院时间(所有 3 项措施的平均差异,0 天,95% CI,0-0 天)。报告的不良反应数量在组间没有差异(2 毫克氟哌啶醇组为 2 [0.3%],安慰剂组为 1 [0.1%])。结论和相关性 在谵妄高危成人中,与安慰剂相比,预防性使用氟哌啶醇并没有提高 28 天的生存率。这些发现不支持预防性使用氟哌啶醇来降低危重成人的死亡率。
更新日期:2018-02-20
中文翻译:

氟哌啶醇对谵妄高危危重患者生存率的影响
重要性 关于在危重成人患者中预防性使用氟哌啶醇的研究结果尚无定论,尤其是在谵妄高危患者中。目的 确定预防性使用氟哌啶醇是否能改善谵妄高危危重成人患者的生存率,谵妄的定义是预计在重症监护室 (ICU) 停留至少 2 天。设计、设置和参与者 随机、双盲、安慰剂对照、研究者驱动的研究,涉及 1789 名在 21 个 ICU 接受治疗的危重成人,在这些 ICU 中,荷兰常规使用预防谵妄的非药物干预措施。预计入住ICU至少一天的没有谵妄的患者被包括在内。招募时间为2013年7月至2016年12月,随访90天,末次随访于2017年3月1日。干预 患者每天接受 3 次静脉内预防性治疗,即 1 毫克(n = 350)或 2 毫克(n = 732)氟哌啶醇或安慰剂(n = 707),由 0.9% 氯化钠组成。主要结果和措施主要结果是患者在 28 天内存活的天数。有 15 个次要结果,包括谵妄发生率、28 天无谵妄和昏迷天数、机械通气持续时间以及 ICU 和住院时间。结果 所有 1789 名随机患者(平均年龄 66.6 岁 [SD,12.6];1099 名男性 [61.4%])完成了研究。1mg 氟哌啶醇组因无效而提前停止。患者在 28 天内存活的中位天数没有差异,2 毫克氟哌啶醇组为 28 天,安慰剂组为 28 天,差异为 0 天(95% CI,0-0;P = . 93) 和 1.003 的风险比 (95% CI, 0.78-1.30, P=.82)。所有 15 项次要结果均无统计学差异。这些包括谵妄发生率(平均差异,1.5%,95% CI,-3.6% 至 6.7%),无谵妄和无昏迷天数(平均差异,0 天,95% CI,0-0 天)和持续时间机械通气、ICU 和住院时间(所有 3 项措施的平均差异,0 天,95% CI,0-0 天)。报告的不良反应数量在组间没有差异(2 毫克氟哌啶醇组为 2 [0.3%],安慰剂组为 1 [0.1%])。结论和相关性 在谵妄高危成人中,与安慰剂相比,预防性使用氟哌啶醇并没有提高 28 天的生存率。这些发现不支持预防性使用氟哌啶醇来降低危重成人的死亡率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号