当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reinventing Fenton Chemistry: Iron Oxychloride Nanosheet for pH-Insensitive H2O2 Activation
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1021/acs.estlett.8b00065 Meng Sun 1 , Chiheng Chu 1 , Fanglan Geng 2 , Xinglin Lu 1 , Jiuhui Qu 3 , John Crittenden 4 , Menachem Elimelech 1 , Jae-Hong Kim 1
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1021/acs.estlett.8b00065 Meng Sun 1 , Chiheng Chu 1 , Fanglan Geng 2 , Xinglin Lu 1 , Jiuhui Qu 3 , John Crittenden 4 , Menachem Elimelech 1 , Jae-Hong Kim 1
Affiliation
|
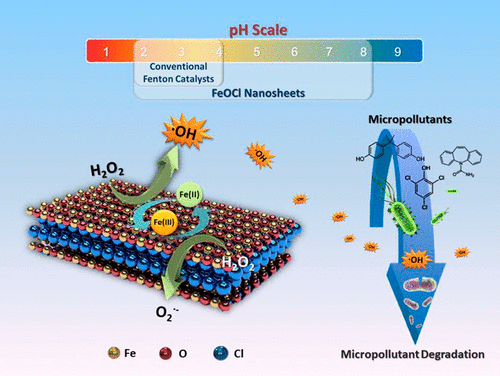
This study intends to reinvent classical Fenton chemistry by enabling the Fe(II)/Fe(III) redox cycle to occur on a newly developed FeOCl nanosheet catalyst for facile hydroxyl radical (•OH) generation from H2O2 activation. This approach overcomes challenges such as low operating pH and large sludge production that have prevented a wider use of otherwise attractive Fenton chemistry for practical water treatment, in particular, for the destruction of recalcitrant pollutants through nonselective oxidation by •OH. We demonstrate that FeOCl catalysts exhibit the highest performance reported in the literature for •OH production and organic pollutant destruction over a wide pH range. We further elucidate the mechanism of rapid conversion between Fe(III) and Fe(II) in FeOCl crystals based on extensive characterizations. Given the low-cost raw material and simple synthesis and regeneration, FeOCl catalysts represent a critical advance toward application of iron-based advanced oxidation in real practice.
中文翻译:

重塑Fenton化学:用于pH不敏感的H 2 O 2活化的三氧化二铁纳米片
这项研究旨在通过使Fe(II)/ Fe(III)氧化还原循环发生在新开发的FeOCl纳米片状催化剂上,以通过H 2 O 2活化产生容易的羟基自由基(• OH)来重塑经典的Fenton化学。这种方法克服了诸如低运行pH值和大量污泥产生的挑战,这些挑战阻止了原本具有吸引力的Fenton化学方法在实际水处理中的广泛应用,尤其是通过• OH的非选择性氧化来破坏顽固性污染物。我们证明FeOCl催化剂具有在文献中报道的最高性能•在很宽的pH范围内产生OH和破坏有机污染物。基于广泛的表征,我们进一步阐明了FeOCl晶体中Fe(III)和Fe(II)之间快速转化的机理。鉴于廉价的原料以及简单的合成和再生,FeOCl催化剂代表了在实际实践中应用铁基高级氧化技术的关键进展。
更新日期:2018-02-21
中文翻译:

重塑Fenton化学:用于pH不敏感的H 2 O 2活化的三氧化二铁纳米片
这项研究旨在通过使Fe(II)/ Fe(III)氧化还原循环发生在新开发的FeOCl纳米片状催化剂上,以通过H 2 O 2活化产生容易的羟基自由基(• OH)来重塑经典的Fenton化学。这种方法克服了诸如低运行pH值和大量污泥产生的挑战,这些挑战阻止了原本具有吸引力的Fenton化学方法在实际水处理中的广泛应用,尤其是通过• OH的非选择性氧化来破坏顽固性污染物。我们证明FeOCl催化剂具有在文献中报道的最高性能•在很宽的pH范围内产生OH和破坏有机污染物。基于广泛的表征,我们进一步阐明了FeOCl晶体中Fe(III)和Fe(II)之间快速转化的机理。鉴于廉价的原料以及简单的合成和再生,FeOCl催化剂代表了在实际实践中应用铁基高级氧化技术的关键进展。









































 京公网安备 11010802027423号
京公网安备 11010802027423号