当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Plasmodium (M)TRAP–Aldolase Interaction Stabilizers Interfering with Sporozoite Motility and Invasion
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acsinfecdis.7b00225 Lauren E. Boucher 1, 2 , Christine S. Hopp 2, 3, 4 , Julianne Mendi Muthinja 5 , Friedrich Frischknecht 5 , Jürgen Bosch 1, 2, 6
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-02-07 00:00:00 , DOI: 10.1021/acsinfecdis.7b00225 Lauren E. Boucher 1, 2 , Christine S. Hopp 2, 3, 4 , Julianne Mendi Muthinja 5 , Friedrich Frischknecht 5 , Jürgen Bosch 1, 2, 6
Affiliation
|
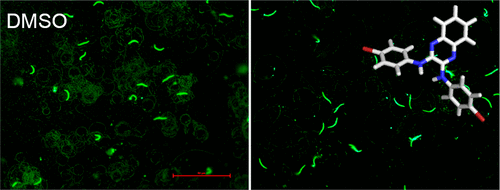
As obligate, intracellular parasites, Plasmodium spp. rely on invasion of host cells in order to replicate and continue their life cycle. The parasite needs to traverse the dermis and endothelium of blood vessels, invade hepatocytes and red blood cells, traverse the mosquito midgut, and enter the salivary glands to continue the cycle of infection and transmission. To traverse and invade cells, the parasite employs an actomyosin motor at the core of a larger invasion machinery complex known as the glideosome. The complex is comprised of multiple protein–protein interactions linking the motor to the internal cytoskeletal network of the parasite and to the extracellular adhesins, which directly contact the host tissue or cell surface. One key interaction is between the cytoplasmic tails of the thrombospondin related anonymous protein (TRAP) and aldolase, a bridging protein to the motor. Here, we present results from screening the Medicines for Malaria Venture (MMV) library of 400 compounds against this key protein–protein interaction. Using a surface plasmon resonance screen, we have identified several compounds that modulate the dynamics of the interaction between TRAP and aldolase. These compounds have been validated in vitro by studying their effects on sporozoite gliding motility and hepatocyte invasion. One of the MMV compounds identified reduced invasion levels by 89% at the lowest concentration tested (16 μM) and severely inhibited gliding at even lower concentrations (5 μM). By targeting protein–protein interactions, we investigated an under-explored area of parasite biology and general drug development, to identify potential antimalarial lead compounds.
中文翻译:

的发现疟原虫(M)TRAP-醛缩酶的相互作用的稳定剂与干扰子孢子运动和侵入
如专性,细胞内寄生虫,疟原虫属。依赖于宿主细胞的入侵才能复制并继续其生命周期。寄生虫需要穿过血管的真皮和内皮,侵入肝细胞和红细胞,穿过蚊子中肠并进入唾液腺以继续感染和传播的循环。为了遍历和侵袭细胞,该寄生虫在一个更大的入侵机械复合体(即滑翔体)的核心处使用放线菌素马达。该复合物由多种蛋白质-蛋白质相互作用组成,将电动机与寄生虫的内部细胞骨架网络以及直接接触宿主组织或细胞表面的细胞外粘附素相连。一个关键的相互作用是血小板反应蛋白相关的匿名蛋白(TRAP)的胞质尾部和醛缩酶之间的结合,醛缩酶是一种连接于运动的蛋白。这里,我们提供了筛选针对这种关键蛋白质相互作用的400种化合物的疟疾药物风险(MMV)库的结果。使用表面等离振子共振筛选,我们已经确定了几种化合物,可以调节TRAP和醛缩酶之间相互作用的动力学。这些化合物已经过验证通过研究它们对子孢子滑行运动和肝细胞侵袭的影响进行体外研究。一种MMV化合物鉴定出在最低测试浓度(16μM)下的侵袭水平降低了89%,并且在更低浓度(5μM)时严重抑制了滑动。通过针对蛋白质之间的相互作用,我们研究了寄生虫生物学和一般药物开发的未充分研究的领域,以确定潜在的抗疟疾先导化合物。
更新日期:2018-02-07
中文翻译:

的发现疟原虫(M)TRAP-醛缩酶的相互作用的稳定剂与干扰子孢子运动和侵入
如专性,细胞内寄生虫,疟原虫属。依赖于宿主细胞的入侵才能复制并继续其生命周期。寄生虫需要穿过血管的真皮和内皮,侵入肝细胞和红细胞,穿过蚊子中肠并进入唾液腺以继续感染和传播的循环。为了遍历和侵袭细胞,该寄生虫在一个更大的入侵机械复合体(即滑翔体)的核心处使用放线菌素马达。该复合物由多种蛋白质-蛋白质相互作用组成,将电动机与寄生虫的内部细胞骨架网络以及直接接触宿主组织或细胞表面的细胞外粘附素相连。一个关键的相互作用是血小板反应蛋白相关的匿名蛋白(TRAP)的胞质尾部和醛缩酶之间的结合,醛缩酶是一种连接于运动的蛋白。这里,我们提供了筛选针对这种关键蛋白质相互作用的400种化合物的疟疾药物风险(MMV)库的结果。使用表面等离振子共振筛选,我们已经确定了几种化合物,可以调节TRAP和醛缩酶之间相互作用的动力学。这些化合物已经过验证通过研究它们对子孢子滑行运动和肝细胞侵袭的影响进行体外研究。一种MMV化合物鉴定出在最低测试浓度(16μM)下的侵袭水平降低了89%,并且在更低浓度(5μM)时严重抑制了滑动。通过针对蛋白质之间的相互作用,我们研究了寄生虫生物学和一般药物开发的未充分研究的领域,以确定潜在的抗疟疾先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号