当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Developments in Glycopeptide Antibiotics.
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-02-19 , DOI: 10.1021/acsinfecdis.7b00258 Mark A T Blaskovich 1, 2 , Karl A Hansford 1, 2 , Mark S Butler 1, 2 , ZhiGuang Jia 1, 2 , Alan E Mark 1, 2 , Matthew A Cooper 1, 2
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-02-19 , DOI: 10.1021/acsinfecdis.7b00258 Mark A T Blaskovich 1, 2 , Karl A Hansford 1, 2 , Mark S Butler 1, 2 , ZhiGuang Jia 1, 2 , Alan E Mark 1, 2 , Matthew A Cooper 1, 2
Affiliation
|
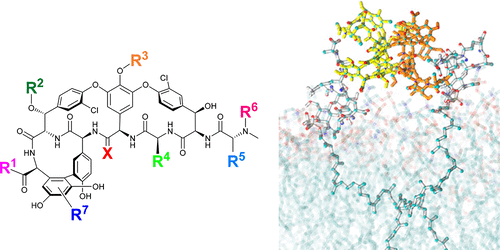
Glycopeptide antibiotics (GPAs) are a key weapon in the fight against drug resistant bacteria, with vancomycin still a mainstream therapy against serious Gram-positive infections more than 50 years after it was first introduced. New, more potent semisynthetic derivatives that have entered the clinic, such as dalbavancin and oritavancin, have superior pharmacokinetic and target engagement profiles that enable successful treatment of vancomycin-resistant infections. In the face of resistance development, with multidrug resistant (MDR) S. pneumoniae and methicillin-resistant Staphylococcus aureus (MRSA) together causing 20-fold more infections than all MDR Gram-negative infections combined, further improvements are desirable to ensure the Gram-positive armamentarium is adequately maintained for future generations. A range of modified glycopeptides has been generated in the past decade via total syntheses, semisynthetic modifications of natural products, or biological engineering. Several of these have undergone extensive characterization with demonstrated in vivo efficacy, good PK/PD profiles, and no reported preclinical toxicity; some may be suitable for formal preclinical development. The natural product monobactam, cephalosporin, and β-lactam antibiotics all spawned multiple generations of commercially and clinically successful semisynthetic derivatives. Similarly, next-generation glycopeptides are now technically well positioned to advance to the clinic, if sufficient funding and market support returns to antibiotic development.
中文翻译:

糖肽抗生素的发展。
糖肽抗生素(GPA)是抗药性细菌的主要武器,万古霉素在首次引入后已有50多年的历史,它仍然是抵抗严重革兰氏阳性感染的主流疗法。进入临床的新的,更有效的半合成衍生物(如达巴万星和奥利万星)具有出色的药代动力学和靶点结合特性,可成功治疗耐万古霉素的感染。面对耐药性的发展,多重耐药性(MDR)肺炎链球菌和耐甲氧西林金黄色葡萄球菌(MRSA)造成的感染比所有MDR革兰氏阴性感染的总和多20倍,因此,需要进一步改进以确保革兰氏阴性菌积极的武器库已为子孙后代充分维持。在过去的十年中,通过总合成,天然产物的半合成修饰或生物工程,已经产生了一系列修饰的糖肽。其中一些已经进行了广泛的表征,具有体内功效,良好的PK / PD分布图,并且没有报道的临床前毒性。有些可能适合正式的临床前开发。天然产物单bactam,头孢菌素和β-内酰胺类抗生素都产生了多代商业上和临床上成功的半合成衍生物。同样,如果抗生素开发得到足够的资金和市场支持,那么下一代糖肽现在在技术上已经可以很好地进入临床。或生物工程。其中一些已经进行了广泛的表征,具有体内功效,良好的PK / PD分布图,并且没有报道的临床前毒性。有些可能适合正式的临床前开发。天然产物单bactam,头孢菌素和β-内酰胺类抗生素都产生了多代商业上和临床上成功的半合成衍生物。同样,如果抗生素开发得到足够的资金和市场支持,那么下一代糖肽现在在技术上已经可以很好地进入临床。或生物工程。其中一些已经进行了广泛的表征,具有体内功效,良好的PK / PD分布图,并且没有报道的临床前毒性。有些可能适合正式的临床前开发。天然产物单bactam,头孢菌素和β-内酰胺类抗生素都产生了多代商业上和临床上成功的半合成衍生物。同样,如果抗生素开发得到足够的资金和市场支持,那么下一代糖肽现在在技术上已经可以很好地进入临床。β-内酰胺类抗生素都产生了多代商业上和临床上成功的半合成衍生物。同样,如果抗生素开发得到足够的资金和市场支持,那么下一代糖肽现在在技术上已经可以很好地进入临床。β-内酰胺类抗生素都产生了多代商业上和临床上成功的半合成衍生物。同样,如果抗生素开发得到足够的资金和市场支持,那么下一代糖肽现在在技术上已经可以很好地进入临床。
更新日期:2018-01-24
中文翻译:

糖肽抗生素的发展。
糖肽抗生素(GPA)是抗药性细菌的主要武器,万古霉素在首次引入后已有50多年的历史,它仍然是抵抗严重革兰氏阳性感染的主流疗法。进入临床的新的,更有效的半合成衍生物(如达巴万星和奥利万星)具有出色的药代动力学和靶点结合特性,可成功治疗耐万古霉素的感染。面对耐药性的发展,多重耐药性(MDR)肺炎链球菌和耐甲氧西林金黄色葡萄球菌(MRSA)造成的感染比所有MDR革兰氏阴性感染的总和多20倍,因此,需要进一步改进以确保革兰氏阴性菌积极的武器库已为子孙后代充分维持。在过去的十年中,通过总合成,天然产物的半合成修饰或生物工程,已经产生了一系列修饰的糖肽。其中一些已经进行了广泛的表征,具有体内功效,良好的PK / PD分布图,并且没有报道的临床前毒性。有些可能适合正式的临床前开发。天然产物单bactam,头孢菌素和β-内酰胺类抗生素都产生了多代商业上和临床上成功的半合成衍生物。同样,如果抗生素开发得到足够的资金和市场支持,那么下一代糖肽现在在技术上已经可以很好地进入临床。或生物工程。其中一些已经进行了广泛的表征,具有体内功效,良好的PK / PD分布图,并且没有报道的临床前毒性。有些可能适合正式的临床前开发。天然产物单bactam,头孢菌素和β-内酰胺类抗生素都产生了多代商业上和临床上成功的半合成衍生物。同样,如果抗生素开发得到足够的资金和市场支持,那么下一代糖肽现在在技术上已经可以很好地进入临床。或生物工程。其中一些已经进行了广泛的表征,具有体内功效,良好的PK / PD分布图,并且没有报道的临床前毒性。有些可能适合正式的临床前开发。天然产物单bactam,头孢菌素和β-内酰胺类抗生素都产生了多代商业上和临床上成功的半合成衍生物。同样,如果抗生素开发得到足够的资金和市场支持,那么下一代糖肽现在在技术上已经可以很好地进入临床。β-内酰胺类抗生素都产生了多代商业上和临床上成功的半合成衍生物。同样,如果抗生素开发得到足够的资金和市场支持,那么下一代糖肽现在在技术上已经可以很好地进入临床。β-内酰胺类抗生素都产生了多代商业上和临床上成功的半合成衍生物。同样,如果抗生素开发得到足够的资金和市场支持,那么下一代糖肽现在在技术上已经可以很好地进入临床。











































 京公网安备 11010802027423号
京公网安备 11010802027423号