当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pharmacological Inhibition of O-GlcNAcase Enhances Autophagy in Brain through an mTOR-Independent Pathway
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1021/acschemneuro.8b00015 Yanping Zhu 1, 2, 3 , Xiaoyang Shan 1 , Farzaneh Safarpour 3, 4 , Nancy Erro Go 5 , Nancy Li 2 , Alice Shan 1 , Mina C. Huang 3, 4 , Matthew Deen 1 , Viktor Holicek 1 , Roger Ashmus 1 , Zarina Madden 1 , Sharon Gorski 2, 3, 5 , Michael A. Silverman 3, 4 , David J. Vocadlo 1, 2, 3
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1021/acschemneuro.8b00015 Yanping Zhu 1, 2, 3 , Xiaoyang Shan 1 , Farzaneh Safarpour 3, 4 , Nancy Erro Go 5 , Nancy Li 2 , Alice Shan 1 , Mina C. Huang 3, 4 , Matthew Deen 1 , Viktor Holicek 1 , Roger Ashmus 1 , Zarina Madden 1 , Sharon Gorski 2, 3, 5 , Michael A. Silverman 3, 4 , David J. Vocadlo 1, 2, 3
Affiliation
|
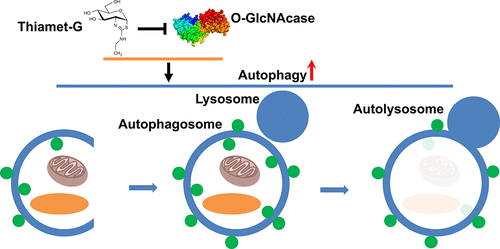
The glycosylation of nucleocytoplasmic proteins with O-linked N-acetylglucosamine residues (O-GlcNAc) is conserved among metazoans and is particularly abundant within brain. O-GlcNAc is involved in diverse cellular processes ranging from the regulation of gene expression to stress response. Moreover, O-GlcNAc is implicated in various diseases including cancers, diabetes, cardiac dysfunction, and neurodegenerative diseases. Pharmacological inhibition of O-GlcNAcase (OGA), the sole enzyme that removes O-GlcNAc, reproducibly slows neurodegeneration in various Alzheimer’s disease (AD) mouse models manifesting either tau or amyloid pathology. These data have stimulated interest in the possibility of using OGA-selective inhibitors as pharmaceuticals to alter the progression of AD. The mechanisms mediating the neuroprotective effects of OGA inhibitors, however, remain poorly understood. Here we show, using a range of methods in neuroblastoma N2a cells, in primary rat neurons, and in mouse brain, that selective OGA inhibitors stimulate autophagy through an mTOR-independent pathway without obvious toxicity. Additionally, OGA inhibition significantly decreased the levels of toxic protein species associated with AD pathogenesis in the JNPL3 tauopathy mouse model as well as the 3×Tg-AD mouse model. These results strongly suggest that OGA inhibitors act within brain through a mechanism involving enhancement of autophagy, which aids the brain in combatting the accumulation of toxic protein species. Our study supports OGA inhibition being a feasible therapeutic strategy for hindering the progression of AD and other neurodegenerative diseases. Moreover, these data suggest more targeted strategies to stimulate autophagy in an mTOR-independent manner may be found within the O-GlcNAc pathway. These findings should aid the advancement of OGA inhibitors within the clinic.
中文翻译:

O-GlcNAcase的药理学抑制作用通过不依赖mTOR的途径增强脑中的自噬
带有O联氮的核质蛋白的糖基化-乙酰氨基葡萄糖残基(O-GlcNAc)在后生动物中是保守的,在大脑中尤其丰富。O-GlcNAc参与了多种细胞过程,从基因表达的调控到应激反应。此外,O-GlcNAc与多种疾病有关,包括癌症,糖尿病,心脏功能障碍和神经退行性疾病。O-GlcNAcase(OGA)的药理抑制作用可去除O-GlcNAc的唯一酶,可重现性地减慢了各种表现出tau或淀粉样蛋白病状的阿尔茨海默氏病(AD)小鼠模型的神经变性。这些数据激发了人们对使用OGA选择性抑制剂作为药物改变AD进展的兴趣。然而,对OGA抑制剂的神经保护作用介导的机制仍知之甚少。我们在这里展示,在神经母细胞瘤N2a细胞,原代大鼠神经元和小鼠大脑中使用多种方法,选择性OGA抑制剂可通过不依赖mTOR的途径刺激自噬,而无明显毒性。此外,在JNPL3 tauopathy小鼠模型以及3xTg-AD小鼠模型中,OGA抑制作用显着降低了与AD发病机理相关的毒性蛋白种类的水平。这些结果强烈表明,OGA抑制剂通过一种涉及自噬增强的机制在大脑中起作用,该机制有助于大脑对抗有毒蛋白质物种的积累。我们的研究支持OGA抑制是阻止AD和其他神经退行性疾病进展的可行治疗策略。而且,这些数据表明,可以在O-GlcNAc途径中发现以mTOR独立的方式刺激自噬的更有针对性的策略。这些发现应有助于临床中OGA抑制剂的发展。
更新日期:2018-02-20
中文翻译:

O-GlcNAcase的药理学抑制作用通过不依赖mTOR的途径增强脑中的自噬
带有O联氮的核质蛋白的糖基化-乙酰氨基葡萄糖残基(O-GlcNAc)在后生动物中是保守的,在大脑中尤其丰富。O-GlcNAc参与了多种细胞过程,从基因表达的调控到应激反应。此外,O-GlcNAc与多种疾病有关,包括癌症,糖尿病,心脏功能障碍和神经退行性疾病。O-GlcNAcase(OGA)的药理抑制作用可去除O-GlcNAc的唯一酶,可重现性地减慢了各种表现出tau或淀粉样蛋白病状的阿尔茨海默氏病(AD)小鼠模型的神经变性。这些数据激发了人们对使用OGA选择性抑制剂作为药物改变AD进展的兴趣。然而,对OGA抑制剂的神经保护作用介导的机制仍知之甚少。我们在这里展示,在神经母细胞瘤N2a细胞,原代大鼠神经元和小鼠大脑中使用多种方法,选择性OGA抑制剂可通过不依赖mTOR的途径刺激自噬,而无明显毒性。此外,在JNPL3 tauopathy小鼠模型以及3xTg-AD小鼠模型中,OGA抑制作用显着降低了与AD发病机理相关的毒性蛋白种类的水平。这些结果强烈表明,OGA抑制剂通过一种涉及自噬增强的机制在大脑中起作用,该机制有助于大脑对抗有毒蛋白质物种的积累。我们的研究支持OGA抑制是阻止AD和其他神经退行性疾病进展的可行治疗策略。而且,这些数据表明,可以在O-GlcNAc途径中发现以mTOR独立的方式刺激自噬的更有针对性的策略。这些发现应有助于临床中OGA抑制剂的发展。



























 京公网安备 11010802027423号
京公网安备 11010802027423号