当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of novel 7-azaspiro[3.5]nonane derivatives as GPR119 agonists
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-02-20 , DOI: 10.1016/j.bmc.2018.02.032 Daisuke Matsuda , Madoka Kawamura , Yohei Kobashi , Fumiyasu Shiozawa , Youichirou Suga , Keiko Fusegi , Shinichi Nishimoto , Kayo Kimura , Masako Miyoshi , Noriko Takayama , Hiroyuki Kakinuma , Norikazu Ohtake
中文翻译:

新型7-氮杂螺[3.5]壬烷衍生物作为GPR119激动剂的设计,合成及生物学评价
更新日期:2018-02-20
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-02-20 , DOI: 10.1016/j.bmc.2018.02.032 Daisuke Matsuda , Madoka Kawamura , Yohei Kobashi , Fumiyasu Shiozawa , Youichirou Suga , Keiko Fusegi , Shinichi Nishimoto , Kayo Kimura , Masako Miyoshi , Noriko Takayama , Hiroyuki Kakinuma , Norikazu Ohtake
|
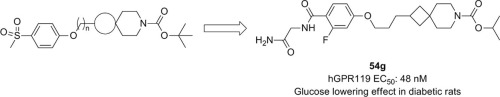
The design and synthesis of a novel class of 7-azaspiro[3.5]nonane GPR119 agonists are described. In this series, optimization of the right piperidine N-capping group (R2) and the left aryl group (R3) led to the identification of compound 54g as a potent GPR119 agonist. Compound 54g showed a desirable PK profile in Sprague-Dawley (SD) rats and a favorable glucose lowering effect in diabetic rats.
中文翻译:

新型7-氮杂螺[3.5]壬烷衍生物作为GPR119激动剂的设计,合成及生物学评价
描述了新型7-氮杂螺[3.5]壬烷GPR119激动剂的设计和合成。在该系列中,右哌啶N封端基团(R 2)和左芳基基团(R 3)的优化导致将化合物54g鉴定为有效的GPR119激动剂。化合物54g在Sprague-Dawley(SD)大鼠中显示出理想的PK分布,在糖尿病大鼠中表现出良好的降糖效果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号