当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Half-Sandwich Arene Ruthenium(II) and Osmium(II) Thiosemicarbazone Complexes: Solution Behavior and Antiproliferative Activity.
Organometallics ( IF 2.5 ) Pub Date : 2018-02-20 , DOI: 10.1021/acs.organomet.7b00875 Anna Gatti 1, 2 , Abraha Habtemariam 2 , Isolda Romero-Canelón 2, 3 , Ji-Inn Song 2 , Bindy Heer 2 , Guy J Clarkson 2 , Dominga Rogolino 1 , Peter J Sadler 2 , Mauro Carcelli 1
Organometallics ( IF 2.5 ) Pub Date : 2018-02-20 , DOI: 10.1021/acs.organomet.7b00875 Anna Gatti 1, 2 , Abraha Habtemariam 2 , Isolda Romero-Canelón 2, 3 , Ji-Inn Song 2 , Bindy Heer 2 , Guy J Clarkson 2 , Dominga Rogolino 1 , Peter J Sadler 2 , Mauro Carcelli 1
Affiliation
|
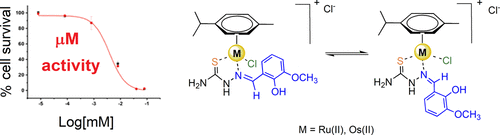
We report the synthesis, characterization, and antiproliferative activity of organo-osmium(II) and organo-ruthenium(II) half-sandwich complexes [(η6-p-cym)Os(L)Cl]Cl (1 and 2) and [(η6-p-cym)Ru(L)Cl]Cl (3 and 4), where L = N-(2-hydroxy)-3-methoxybenzylidenethiosemicarbazide (L1) or N-(2,3-dihydroxybenzylidene)-3-phenylthiosemicarbazide (L2), respectively. X-ray crystallography showed that all four complexes possess half-sandwich pseudo-octahedral "three-legged piano-stool" structures, with a neutral N,S-chelating thiosemicarbazone ligand and a terminal chloride occupying three coordination positions. In methanol, E/Z isomerization of the coordinated thiosemicarbazone ligand was observed, while in an aprotic solvent like acetone, partial dissociation of the ligand occurs, reaching complete displacement in a more coordinating solvent like DMSO. In general, the complexes exhibited good activity toward A2780 ovarian, A2780Cis cisplatin-resistant ovarian, A549 lung, HCT116 colon, and PC3 prostate cancer cells. In particular, ruthenium complex 3 does not present cross-resistance with the clinical drug cisplatin in the A2780 human ovarian cancer cell line. The complexes were more active than the free thiosemicarbazone ligands, especially in A549 and HCT116 cells with potency improvements of up to 20-fold between organic ligand L1 and ruthenium complex 1.
中文翻译:

半夹心芳烃钌 (II) 和锇 (II) 缩氨基硫脲复合物:溶液行为和抗增殖活性。
我们报告了有机锇(II)和有机钌(II)半夹心配合物[(η6-p-cym)Os(L)Cl]Cl(1和2)和[的合成、表征和抗增殖活性。 (η6-p-cym)Ru(L)Cl]Cl(3 和 4),其中 L = N-(2-羟基)-3-甲氧基亚苄基氨基硫脲 (L1) 或 N-(2,3-二羟基亚苄基)-3-分别为氨基硫脲(L2)。 X射线晶体学表明,所有四种配合物均具有半夹心假八面体“三足琴凳”结构,其中中性N,S螯合缩氨基硫脲配体和末端氯占据三个配位。在甲醇中,观察到配位缩氨基硫脲配体的 E/Z 异构化,而在丙酮等非质子溶剂中,配体发生部分解离,在更配位的溶剂(如 DMSO)中达到完全置换。一般来说,该复合物对 A2780 卵巢癌细胞、A2780Cis 顺铂耐药卵巢癌细胞、A549 肺癌细胞、HCT116 结肠癌细胞和 PC3 前列腺癌细胞表现出良好的活性。特别是,钌络合物3在A2780人卵巢癌细胞系中不与临床药物顺铂呈现交叉耐药性。这些配合物比游离的缩氨基硫脲配体更具活性,特别是在 A549 和 HCT116 细胞中,有机配体 L1 和钌配合物 1 之间的效力提高高达 20 倍。
更新日期:2018-02-21
中文翻译:

半夹心芳烃钌 (II) 和锇 (II) 缩氨基硫脲复合物:溶液行为和抗增殖活性。
我们报告了有机锇(II)和有机钌(II)半夹心配合物[(η6-p-cym)Os(L)Cl]Cl(1和2)和[的合成、表征和抗增殖活性。 (η6-p-cym)Ru(L)Cl]Cl(3 和 4),其中 L = N-(2-羟基)-3-甲氧基亚苄基氨基硫脲 (L1) 或 N-(2,3-二羟基亚苄基)-3-分别为氨基硫脲(L2)。 X射线晶体学表明,所有四种配合物均具有半夹心假八面体“三足琴凳”结构,其中中性N,S螯合缩氨基硫脲配体和末端氯占据三个配位。在甲醇中,观察到配位缩氨基硫脲配体的 E/Z 异构化,而在丙酮等非质子溶剂中,配体发生部分解离,在更配位的溶剂(如 DMSO)中达到完全置换。一般来说,该复合物对 A2780 卵巢癌细胞、A2780Cis 顺铂耐药卵巢癌细胞、A549 肺癌细胞、HCT116 结肠癌细胞和 PC3 前列腺癌细胞表现出良好的活性。特别是,钌络合物3在A2780人卵巢癌细胞系中不与临床药物顺铂呈现交叉耐药性。这些配合物比游离的缩氨基硫脲配体更具活性,特别是在 A549 和 HCT116 细胞中,有机配体 L1 和钌配合物 1 之间的效力提高高达 20 倍。











































 京公网安备 11010802027423号
京公网安备 11010802027423号