Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible Surface Engineering via Nitrone-Mediated Radical Coupling
Langmuir ( IF 3.7 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acs.langmuir.7b03167 Joachim Laun , Wouter Marchal , Vanessa Trouillet , Alexander Welle , An Hardy , Marlies K. Van Bael , Christopher Barner-Kowollik 1, 2 , Tanja Junkers
Langmuir ( IF 3.7 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1021/acs.langmuir.7b03167 Joachim Laun , Wouter Marchal , Vanessa Trouillet , Alexander Welle , An Hardy , Marlies K. Van Bael , Christopher Barner-Kowollik 1, 2 , Tanja Junkers
Affiliation
|
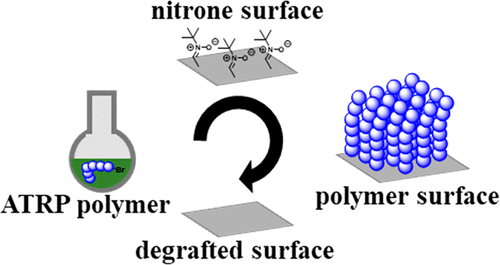
Efficient and simple polymer conjugation reactions are critical for introducing functionalities on surfaces. For polymer surface grafting, postpolymerization modifications are often required, which can impose a significant synthetic hurdle. Here, we report two strategies that allow for reversible surface engineering via nitrone-mediated radical coupling (NMRC). Macroradicals stemming from the activation of polymers generated by copper-mediated radical polymerization are grafted via radical trapping with a surface-immobilized nitrone or a solution-borne nitrone. Since the product of NMRC coupling features an alkoxyamine linker, the grafting reactions can be reversed or chain insertions can be performed via nitroxide-mediated polymerization (NMP). Poly(n-butyl acrylate) (Mn = 1570 g·mol–1, D̵ = 1.12) with a bromine terminus was reversibly grafted to planar silicon substrates or silica nanoparticles as successfully evidenced via X-ray photoelectron spectroscopy (XPS), time-of-flight secondary ion mass spectrometry, and grazing angle attenuated total reflection Fourier-transform infrared spectroscopy (GAATR-FTIR). NMP chain insertions of styrene are evidenced via GAATR-FTIR. On silica nanoparticles, an NMRC grafting density of close to 0.21 chains per nm2 was determined by dynamic light scattering and thermogravimetric analysis. Concomitantly, a simple way to decorate particles with nitroxide radicals with precise control over the radical concentration is introduced. Silica microparticles and zinc oxide, barium titanate, and silicon nanoparticles were successfully functionalized.
中文翻译:

通过Nitrone介导的自由基偶联进行可逆表面工程
高效简单的聚合物共轭反应对于在表面引入功能至关重要。对于聚合物表面接枝,通常需要进行后聚合改性,这可能会带来很大的合成障碍。在这里,我们报告了两种策略,可通过硝酮介导的自由基偶联(NMRC)进行可逆的表面工程。由铜介导的自由基聚合反应产生的聚合物活化而产生的大自由基,通过自由基捕获与表面固定的硝酮或溶液型硝酮接枝。由于NMRC偶联的产物具有烷氧基胺连接基,因此可以逆转接枝反应或可以通过氮氧化物介导的聚合反应(NMP)进行链插入。聚(丙烯酸正丁酯)(M nX射线光电子能谱(XPS),飞行时间二次离子质谱法和X射线光电子能谱法成功地证明,具有1570 g·mol –1的D̵ = 1.12 (D̵ = 1.12)和溴的末端被可逆地接枝到平面硅基底或二氧化硅纳米颗粒上。掠角衰减全反射傅立叶变换红外光谱(GAATR-FTIR)。苯乙烯的NMP链插入通过GAATR-FTIR证明。在二氧化硅纳米颗粒上,NMRC接枝密度接近0.21链/ nm 2通过动态光散射和热重分析确定。因此,引入了一种用氮氧化物自由基修饰颗粒并精确控制自由基浓度的简单方法。二氧化硅微粒和氧化锌,钛酸钡和硅纳米粒子已成功实现功能化。
更新日期:2018-02-19
中文翻译:

通过Nitrone介导的自由基偶联进行可逆表面工程
高效简单的聚合物共轭反应对于在表面引入功能至关重要。对于聚合物表面接枝,通常需要进行后聚合改性,这可能会带来很大的合成障碍。在这里,我们报告了两种策略,可通过硝酮介导的自由基偶联(NMRC)进行可逆的表面工程。由铜介导的自由基聚合反应产生的聚合物活化而产生的大自由基,通过自由基捕获与表面固定的硝酮或溶液型硝酮接枝。由于NMRC偶联的产物具有烷氧基胺连接基,因此可以逆转接枝反应或可以通过氮氧化物介导的聚合反应(NMP)进行链插入。聚(丙烯酸正丁酯)(M nX射线光电子能谱(XPS),飞行时间二次离子质谱法和X射线光电子能谱法成功地证明,具有1570 g·mol –1的D̵ = 1.12 (D̵ = 1.12)和溴的末端被可逆地接枝到平面硅基底或二氧化硅纳米颗粒上。掠角衰减全反射傅立叶变换红外光谱(GAATR-FTIR)。苯乙烯的NMP链插入通过GAATR-FTIR证明。在二氧化硅纳米颗粒上,NMRC接枝密度接近0.21链/ nm 2通过动态光散射和热重分析确定。因此,引入了一种用氮氧化物自由基修饰颗粒并精确控制自由基浓度的简单方法。二氧化硅微粒和氧化锌,钛酸钡和硅纳米粒子已成功实现功能化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号