Journal of Controlled Release ( IF 10.5 ) Pub Date : 2018-02-20 , DOI: 10.1016/j.jconrel.2018.02.024 Victor Jeannot , Cony Gauche , Silvia Mazzaferro , Morgane Couvet , Laetitia Vanwonterghem , Maxime Henry , Chloé Didier , Julien Vollaire , Véronique Josserand , Jean-Luc Coll , Christophe Schatz , Sébastien Lecommandoux , Amandine Hurbin
|
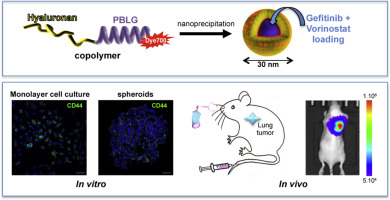
Combinations of therapeutic agents could synergistically enhance the response of lung cancer cells. Co-delivery systems capable of transporting chemotherapeutics with different physicochemical properties and with the simultaneous release of drugs remain elusive. Here, we assess the ability of nanoparticles of 30-nm diameter obtained from the self-assembly of hyaluronan-based copolymer targeting CD44 receptors to encapsulate both gefitinib and vorinostat for effective combinational lung cancer treatment. Drug loading was performed by nanoprecipitation. Drug release experiments showed a slow release of both drugs after 5 days. Using two- and three-dimensional lung adenocarcinoma cell cultures, we observed that the nanoparticles were mostly found at the periphery of the CD44-expressing spheroids. These drug-loaded nanoparticles were as cytotoxic as free drugs in the two- and three-dimensional systems and toxicity was due to apoptosis induction. In mouse models, intravenous injection of hyaluronan-based nanoparticles showed a selective delivery to subcutaneous CD44-overexpressing tumors, despite a significant liver capture. In addition, the systemic toxicity of the free drugs was reduced by their co-delivery using the nanoparticles. Finally, intrapulmonary administration of drug-loaded nanoparticles, to avoid a possible hepatic toxicity due to their accumulation in the liver, showed a stronger inhibition of orthotopic lung tumor growth compared to free drugs. In conclusion, hyaluronan-based nanoparticles provide active targeting partially mediated by CD44, less-toxic drug release and improved antitumor efficiency.
中文翻译:

透明质酸基纳米颗粒在肺癌中药物共同给药的抗肿瘤功效
治疗剂的组合可以协同增强肺癌细胞的反应。能够运送具有不同理化性质的化学治疗剂并同时释放药物的共同给药系统仍然难以捉摸。在这里,我们评估了从透明质酸共聚物靶向CD44受体的自组装获得的直径30 nm的纳米颗粒封装吉非替尼和伏立诺他的有效联合肺癌治疗的能力。通过纳米沉淀进行药物加载。药物释放实验显示5天后两种药物的缓慢释放。使用二维和三维肺腺癌细胞培养,我们观察到纳米颗粒主要存在于表达CD44的球体的外围。这些载有药物的纳米颗粒在二维和三维系统中的细胞毒性与游离药物一样,并且毒性归因于细胞凋亡的诱导。在小鼠模型中,尽管有明显的肝脏捕获,静脉注射透明质酸基纳米颗粒显示选择性递送至过表达CD44的皮下肿瘤。另外,通过使用纳米颗粒共递送,减少了游离药物的全身毒性。最后,与游离药物相比,肺内给药载药的纳米颗粒避免了由于其在肝脏中的积累而可能引起的肝毒性,显示出对原位肺肿瘤生长的抑制作用更强。总之,透明质酸基纳米颗粒可提供部分由CD44介导的活性靶向,低毒药物释放和提高的抗肿瘤效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号