当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conformationally restricted benzothienoazepine respiratory syncytial virus inhibitors: their synthesis, structural analysis and biological activities†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1039/c8md00033f Euan A F Fordyce 1 , S Fraser Hunt 1 , Damien Crepin 1 , Stuart T Onions 1 , Guillaume F Parra 1 , Chris J Sleigh 1 , John King-Underwood 2 , Harry Finch 3 , John Murray 3
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-02-20 00:00:00 , DOI: 10.1039/c8md00033f Euan A F Fordyce 1 , S Fraser Hunt 1 , Damien Crepin 1 , Stuart T Onions 1 , Guillaume F Parra 1 , Chris J Sleigh 1 , John King-Underwood 2 , Harry Finch 3 , John Murray 3
Affiliation
|
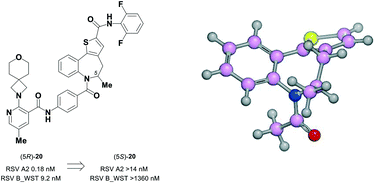
Atropisomeric drug substances are known to have different biological properties. Compounds containing the N-benzoylbenzazepine motif have been shown to exhibit energetically restricted rotation around the Ar(CO)N axis. Herein we report, for the first time, the synthesis, physical characterisation and anti-viral profiles of a series of C-4 and C-5 methylated thieno-benzazepines. NMR analysis reveals that incorporation of a single additional substituent at either of these loci influences the conformational dynamics of the azepine ring system. In the case of the C-5 alkyl analogues, the influence of the new stereocentre is so pronounced that its absolute configuration determines which unique atropisomer is obtained following the generation of the benzazepine nucleus. Screening of the alkylated derivatives for their anti-respiratory syncytial virus (RSV) activity indicates that the desired viral pathogenicity is strongly associated with the conformation adopted by the modified tricyclic scaffolds. This is particularly evident in the case of the C-5 homologues in which one atropisomer was found to be potently active and the other essentially inert. These results provide compelling evidence that we have determined the bioactive conformation shared by RSV inhibitors that employ the thienobenazapine nucleus as their core molecular architecture. Furthermore, the understanding obtained from these studies may make it possible to design improved agents against RSV infection in the future.
中文翻译:

构象受限的苯并噻吩并氮杂呼吸道合胞病毒抑制剂:它们的合成、结构分析和生物活性†
已知阻转异构药物物质具有不同的生物学特性。含有N的化合物-苯甲酰基苯并氮杂基序已显示出围绕 Ar(CO)N 轴的能量受限旋转。在此,我们首次报道了一系列 C-4 和 C-5 甲基化噻吩并苯并氮杂的合成、物理表征和抗病毒谱。NMR 分析表明,在这些基因座中的任何一个处加入一个额外的取代基会影响氮杂环系统的构象动力学。在 C-5 烷基类似物的情况下,新立体中心的影响非常明显,以至于它的绝对构型决定了在生成苯并氮杂核后获得哪种独特的阻转异构体。筛选烷基化衍生物的抗呼吸道合胞病毒 (RSV) 活性表明所需的病毒致病性与修饰的三环支架所采用的构象密切相关。这在 C-5 同系物的情况下尤其明显,其中一种阻转异构体被发现是有效的活性而另一种基本上是惰性的。这些结果提供了令人信服的证据,证明我们已经确定了 RSV 抑制剂共有的生物活性构象,这些抑制剂采用噻吩苯氮平核作为其核心分子结构。此外,从这些研究中获得的理解可能使未来设计改进的抗 RSV 感染药物成为可能。这在 C-5 同系物的情况下尤其明显,其中一种阻转异构体被发现是有效的活性而另一种基本上是惰性的。这些结果提供了令人信服的证据,证明我们已经确定了 RSV 抑制剂共有的生物活性构象,这些抑制剂采用噻吩苯氮平核作为其核心分子结构。此外,从这些研究中获得的理解可能使未来设计改进的抗 RSV 感染药物成为可能。这在 C-5 同系物的情况下尤其明显,其中发现一种阻转异构体具有有效活性而另一种基本上是惰性的。这些结果提供了令人信服的证据,证明我们已经确定了 RSV 抑制剂共有的生物活性构象,这些抑制剂采用噻吩苯氮平核作为其核心分子结构。此外,从这些研究中获得的理解可能使未来设计改进的抗 RSV 感染药物成为可能。
更新日期:2018-02-20
中文翻译:

构象受限的苯并噻吩并氮杂呼吸道合胞病毒抑制剂:它们的合成、结构分析和生物活性†
已知阻转异构药物物质具有不同的生物学特性。含有N的化合物-苯甲酰基苯并氮杂基序已显示出围绕 Ar(CO)N 轴的能量受限旋转。在此,我们首次报道了一系列 C-4 和 C-5 甲基化噻吩并苯并氮杂的合成、物理表征和抗病毒谱。NMR 分析表明,在这些基因座中的任何一个处加入一个额外的取代基会影响氮杂环系统的构象动力学。在 C-5 烷基类似物的情况下,新立体中心的影响非常明显,以至于它的绝对构型决定了在生成苯并氮杂核后获得哪种独特的阻转异构体。筛选烷基化衍生物的抗呼吸道合胞病毒 (RSV) 活性表明所需的病毒致病性与修饰的三环支架所采用的构象密切相关。这在 C-5 同系物的情况下尤其明显,其中一种阻转异构体被发现是有效的活性而另一种基本上是惰性的。这些结果提供了令人信服的证据,证明我们已经确定了 RSV 抑制剂共有的生物活性构象,这些抑制剂采用噻吩苯氮平核作为其核心分子结构。此外,从这些研究中获得的理解可能使未来设计改进的抗 RSV 感染药物成为可能。这在 C-5 同系物的情况下尤其明显,其中一种阻转异构体被发现是有效的活性而另一种基本上是惰性的。这些结果提供了令人信服的证据,证明我们已经确定了 RSV 抑制剂共有的生物活性构象,这些抑制剂采用噻吩苯氮平核作为其核心分子结构。此外,从这些研究中获得的理解可能使未来设计改进的抗 RSV 感染药物成为可能。这在 C-5 同系物的情况下尤其明显,其中发现一种阻转异构体具有有效活性而另一种基本上是惰性的。这些结果提供了令人信服的证据,证明我们已经确定了 RSV 抑制剂共有的生物活性构象,这些抑制剂采用噻吩苯氮平核作为其核心分子结构。此外,从这些研究中获得的理解可能使未来设计改进的抗 RSV 感染药物成为可能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号