当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold-catalyzed [4+3]- and [4+2]-annulations of 3-en-1-ynamides with isoxazoles via novel 6π-electrocyclizations of 3-azahepta trienyl cations†
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1039/c8sc00232k Sovan Sundar Giri 1, 2, 3, 4 , Rai-Shung Liu 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1039/c8sc00232k Sovan Sundar Giri 1, 2, 3, 4 , Rai-Shung Liu 1, 2, 3, 4
Affiliation
|
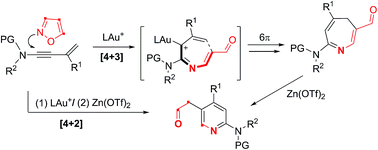
New gold-catalyzed [4+3]-annulations of 3-en-1-ynamides with isoxazoles afford 4H-azepines efficiently; this process involves 6π electrocyclizations of gold-stabilized 3-azaheptatrienyl cations. In the presence of Zn(OTf)2, the resulting 4H-azepines undergo skeletal rearrangement to furnish substituted pyridine derivatives. We subsequently develop new catalytic [4+2]-annulations between the same 3-en-1-ynamides and isoxazoles to deliver substituted pyridine products using Au(I)/Zn(II) catalysts. This work reports the first success of the 6π electrocyclizations of heptatrienyl cations that are unprecedented in literature reports.
中文翻译:

金催化 3-en-1-ynamides 与异恶唑通过 3-azahepta 三烯基阳离子的新型 6π-电环化反应†
新的金催化的 3-en-1-ynamides 与异恶唑的 [4+3]-环化反应有效地产生 4 H-氮杂卓类化合物;该过程涉及金稳定的 3-氮杂七三烯基阳离子的 6π 电环化。在Zn(OTf) 2存在下,所得4 H-氮杂环庚烷发生骨架重排,形成取代的吡啶衍生物。随后,我们在相同的 3-en-1-ynamides 和异恶唑之间开发了新的催化 [4+2]- 环化,以使用 Au( I )/Zn( II ) 催化剂提供取代的吡啶产物。这项工作首次成功实现了庚三烯基阳离子的 6π 电环化,这在文献报道中是前所未有的。
更新日期:2018-02-19
中文翻译:

金催化 3-en-1-ynamides 与异恶唑通过 3-azahepta 三烯基阳离子的新型 6π-电环化反应†
新的金催化的 3-en-1-ynamides 与异恶唑的 [4+3]-环化反应有效地产生 4 H-氮杂卓类化合物;该过程涉及金稳定的 3-氮杂七三烯基阳离子的 6π 电环化。在Zn(OTf) 2存在下,所得4 H-氮杂环庚烷发生骨架重排,形成取代的吡啶衍生物。随后,我们在相同的 3-en-1-ynamides 和异恶唑之间开发了新的催化 [4+2]- 环化,以使用 Au( I )/Zn( II ) 催化剂提供取代的吡啶产物。这项工作首次成功实现了庚三烯基阳离子的 6π 电环化,这在文献报道中是前所未有的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号