当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An unusual stereoretentive 1,3-quaternary carbon shift resulting in an enantioselective RhII-catalyzed formal [4+1]-cycloaddition between diazo compounds and vinyl ketenes†
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1039/c8sc00020d Kevin X Rodriguez 1 , Tara C Pilato 1 , Brandon L Ashfeld 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-19 00:00:00 , DOI: 10.1039/c8sc00020d Kevin X Rodriguez 1 , Tara C Pilato 1 , Brandon L Ashfeld 1
Affiliation
|
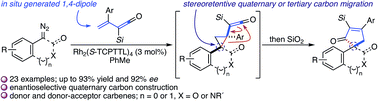
Enantioselective quaternary carbon construction in the assembly of cyclopentenones employing a RhII-catalyzed, formal [4+1]-cycloaddition is described. A Rh2(S-TCPTTL)4-catalyzed cyclopropanation of a vinyl ketene with a disubstituted diazo compound initiates a stereoretentive, accelerated ring expansion to provide the cycloadduct in good to excellent yields and enantioselectivity.
中文翻译:

一种不寻常的立体保留 1,3-季碳位移,导致重氮化合物和乙烯基烯酮之间发生对映选择性 RhII 催化的正式 [4+1]-环加成†
描述了采用 Rh II催化的正式 [4+1]-环加成反应组装环戊烯酮时的对映选择性季碳结构。Rh 2 ( S -TCPTTL) 4催化的乙烯基烯酮与二取代的重氮化合物的环丙烷化引发立构保留、加速环扩张,从而以良好至优异的产率和对映选择性提供环加合物。
更新日期:2018-02-19
中文翻译:

一种不寻常的立体保留 1,3-季碳位移,导致重氮化合物和乙烯基烯酮之间发生对映选择性 RhII 催化的正式 [4+1]-环加成†
描述了采用 Rh II催化的正式 [4+1]-环加成反应组装环戊烯酮时的对映选择性季碳结构。Rh 2 ( S -TCPTTL) 4催化的乙烯基烯酮与二取代的重氮化合物的环丙烷化引发立构保留、加速环扩张,从而以良好至优异的产率和对映选择性提供环加合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号