当前位置:
X-MOL 学术
›
PLOS Pathog.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interactome analysis of the lymphocytic choriomeningitis virus nucleoprotein in infected cells reveals ATPase Na+/K+ transporting subunit Alpha 1 and prohibitin as host-cell factors involved in the life cycle of mammarenaviruses.
PLoS Pathogens ( IF 5.5 ) Pub Date : 2018-02-20 , DOI: 10.1371/journal.ppat.1006892 Masaharu Iwasaki 1 , Petra Minder 1 , Yíngyún Caì 2 , Jens H Kuhn 2 , John R Yates 3 , Bruce E Torbett 1 , Juan C de la Torre 1
PLoS Pathogens ( IF 5.5 ) Pub Date : 2018-02-20 , DOI: 10.1371/journal.ppat.1006892 Masaharu Iwasaki 1 , Petra Minder 1 , Yíngyún Caì 2 , Jens H Kuhn 2 , John R Yates 3 , Bruce E Torbett 1 , Juan C de la Torre 1
Affiliation
|
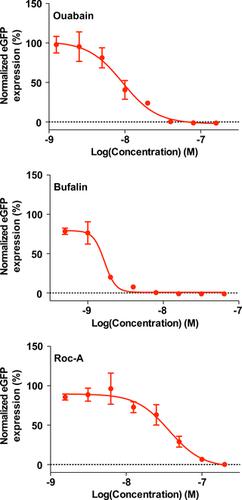
Several mammalian arenaviruses (mammarenaviruses) cause hemorrhagic fevers in humans and pose serious public health concerns in their endemic regions. Additionally, mounting evidence indicates that the worldwide-distributed, prototypic mammarenavirus, lymphocytic choriomeningitis virus (LCMV), is a neglected human pathogen of clinical significance. Concerns about human-pathogenic mammarenaviruses are exacerbated by of the lack of licensed vaccines, and current anti-mammarenavirus therapy is limited to off-label use of ribavirin that is only partially effective. Detailed understanding of virus/host-cell interactions may facilitate the development of novel anti-mammarenavirus strategies by targeting components of the host-cell machinery that are required for efficient virus multiplication. Here we document the generation of a recombinant LCMV encoding a nucleoprotein (NP) containing an affinity tag (rLCMV/Strep-NP) and its use to capture the NP-interactome in infected cells. Our proteomic approach combined with genetics and pharmacological validation assays identified ATPase Na+/K+ transporting subunit alpha 1 (ATP1A1) and prohibitin (PHB) as pro-viral factors. Cell-based assays revealed that ATP1A1 and PHB are involved in different steps of the virus life cycle. Accordingly, we observed a synergistic inhibitory effect on LCMV multiplication with a combination of ATP1A1 and PHB inhibitors. We show that ATP1A1 inhibitors suppress multiplication of Lassa virus and Candid#1, a live-attenuated vaccine strain of Junín virus, suggesting that the requirement of ATP1A1 in virus multiplication is conserved among genetically distantly related mammarenaviruses. Our findings suggest that clinically approved inhibitors of ATP1A1, like digoxin, could be repurposed to treat infections by mammarenaviruses pathogenic for humans.
中文翻译:

对感染细胞中淋巴细胞脉络膜脑膜炎病毒核蛋白的相互作用组分析揭示了 ATP 酶 Na+/K+ 转运亚基 Alpha 1 和抑制素作为参与哺乳动物病毒生命周期的宿主细胞因子。
几种哺乳动物沙粒病毒(mammarenaviruses)会引起人类出血热,并在其流行地区造成严重的公共卫生问题。此外,越来越多的证据表明,全球分布的原型乳房病毒、淋巴细胞性脉络膜脑膜炎病毒(LCMV)是一种被忽视的具有临床意义的人类病原体。由于缺乏获得许可的疫苗,加剧了对人类致病性乳腺病毒的担忧,并且目前的抗乳腺病毒治疗仅限于超说明书使用利巴韦林,而利巴韦林仅部分有效。对病毒/宿主细胞相互作用的详细了解可能会通过针对有效病毒增殖所需的宿主细胞机制的组件来促进新型抗乳房病毒策略的开发。在这里,我们记录了编码含有亲和标签(rLCMV/Strep-NP)的核蛋白(NP)的重组 LCMV 的生成及其用于捕获受感染细胞中的 NP 相互作用组的用途。我们的蛋白质组学方法结合遗传学和药理学验证测定,确定 ATP 酶 Na+/K+ 转运亚基 α 1 (ATP1A1) 和抑制素 (PHB) 为促病毒因子。基于细胞的检测表明 ATP1A1 和 PHB 参与病毒生命周期的不同步骤。因此,我们观察到 ATP1A1 和 PHB 抑制剂的组合对 LCMV 增殖具有协同抑制作用。我们发现 ATP1A1 抑制剂可抑制拉沙病毒和胡宁病毒减毒活疫苗株 Candid#1 的增殖,这表明病毒增殖中对 ATP1A1 的需求在遗传上关系较远的乳腺病毒中是保守的。 我们的研究结果表明,临床批准的 ATP1A1 抑制剂(如地高辛)可以重新用于治疗人类致病性乳腺病毒感染。
更新日期:2018-02-21
中文翻译:

对感染细胞中淋巴细胞脉络膜脑膜炎病毒核蛋白的相互作用组分析揭示了 ATP 酶 Na+/K+ 转运亚基 Alpha 1 和抑制素作为参与哺乳动物病毒生命周期的宿主细胞因子。
几种哺乳动物沙粒病毒(mammarenaviruses)会引起人类出血热,并在其流行地区造成严重的公共卫生问题。此外,越来越多的证据表明,全球分布的原型乳房病毒、淋巴细胞性脉络膜脑膜炎病毒(LCMV)是一种被忽视的具有临床意义的人类病原体。由于缺乏获得许可的疫苗,加剧了对人类致病性乳腺病毒的担忧,并且目前的抗乳腺病毒治疗仅限于超说明书使用利巴韦林,而利巴韦林仅部分有效。对病毒/宿主细胞相互作用的详细了解可能会通过针对有效病毒增殖所需的宿主细胞机制的组件来促进新型抗乳房病毒策略的开发。在这里,我们记录了编码含有亲和标签(rLCMV/Strep-NP)的核蛋白(NP)的重组 LCMV 的生成及其用于捕获受感染细胞中的 NP 相互作用组的用途。我们的蛋白质组学方法结合遗传学和药理学验证测定,确定 ATP 酶 Na+/K+ 转运亚基 α 1 (ATP1A1) 和抑制素 (PHB) 为促病毒因子。基于细胞的检测表明 ATP1A1 和 PHB 参与病毒生命周期的不同步骤。因此,我们观察到 ATP1A1 和 PHB 抑制剂的组合对 LCMV 增殖具有协同抑制作用。我们发现 ATP1A1 抑制剂可抑制拉沙病毒和胡宁病毒减毒活疫苗株 Candid#1 的增殖,这表明病毒增殖中对 ATP1A1 的需求在遗传上关系较远的乳腺病毒中是保守的。 我们的研究结果表明,临床批准的 ATP1A1 抑制剂(如地高辛)可以重新用于治疗人类致病性乳腺病毒感染。











































 京公网安备 11010802027423号
京公网安备 11010802027423号