Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-02-19 , DOI: 10.1016/j.jinorgbio.2018.02.011 Hui-Min Cheng , Hong Yuan , Xiao-Juan Wang , Jia-Kun Xu , Shu-Qin Gao , Ge-Bo Wen , Xiangshi Tan , Ying-Wu Lin
|
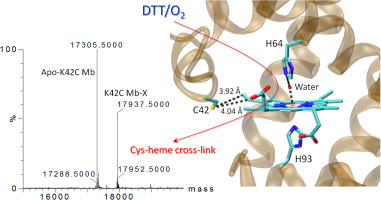
The structure and function of heme proteins are regulated by diverse post-translational modifications including heme-protein cross-links, with the underlying mechanisms not well understood. In this study, we introduced a Cys (K42C) close to the heme 4-vinyl group in sperm whale myoglobin (Mb) and solved its X-ray crystal structure. Interestingly, we found that K42C Mb can partially form a Cys-heme cross-link (termed K42C Mb-X) under dithiothreitol-induced reductive conditions in presence of O2, as suggested by guanidine hydrochloride-induced unfolding and heme extraction studies. Mass spectrometry (MS) studies, together with trypsin digestion studies, further indicated that a thioether bond is formed between Cys42 and the heme 4-vinyl group with an additional mass of 16 Da, likely due to hydroxylation of the α‑carbon. We then proposed a plausible mechanism for the formation of the novel Cys-heme cross-link based on MS, kinetic UV–vis and electron paramagnetic resonance (EPR) studies. Moreover, the Cys-heme cross-link was shown to fine-tune the protein reactivity toward activation of H2O2. This study provides valuable insights into the post-translational modification of heme proteins, and also suggests that the Cys-heme cross-link can be induced to form in vitro, making it useful for design of new heme proteins with a non-dissociable heme and improved functions.
中文翻译:

分子氧还原条件下K42C肌红蛋白中半胱氨酸血红素交联的形成
血红素蛋白的结构和功能受包括血红素蛋白交联在内的多种翻译后修饰的调控,其潜在机理尚不清楚。在这项研究中,我们在抹香鲸肌红蛋白(Mb)中引入了一个靠近血红素4-乙烯基的Cys(K42C),并解决了其X射线晶体结构。有趣的是,我们发现在O 2的存在下,二硫苏糖醇诱导的还原条件下,K42C Mb可以部分形成Cys-血红素交联键(称为K42C Mb-X)。,如盐酸胍诱导的展开和血红素提取研究所建议。质谱(MS)研究以及胰蛋白酶消化研究进一步表明,Cys42与血红素4-乙烯基之间形成硫醚键,其附加质量为16 Da,这可能是由于α-碳的羟基化所致。然后,我们根据质谱,动力学紫外可见和电子顺磁共振(EPR)研究提出了一种新型Cys-血红素交联形成的合理机制。此外,Cys-血红素交联显示出可微调蛋白质对H 2 O 2活化的反应性。这项研究提供了对血红素蛋白翻译后修饰的有价值的见解,并且还暗示可以诱导Cys-血红素交联形成体外,使其可用于设计具有不可解离血红素和改善功能的新型血红素蛋白。











































 京公网安备 11010802027423号
京公网安备 11010802027423号